Abstract
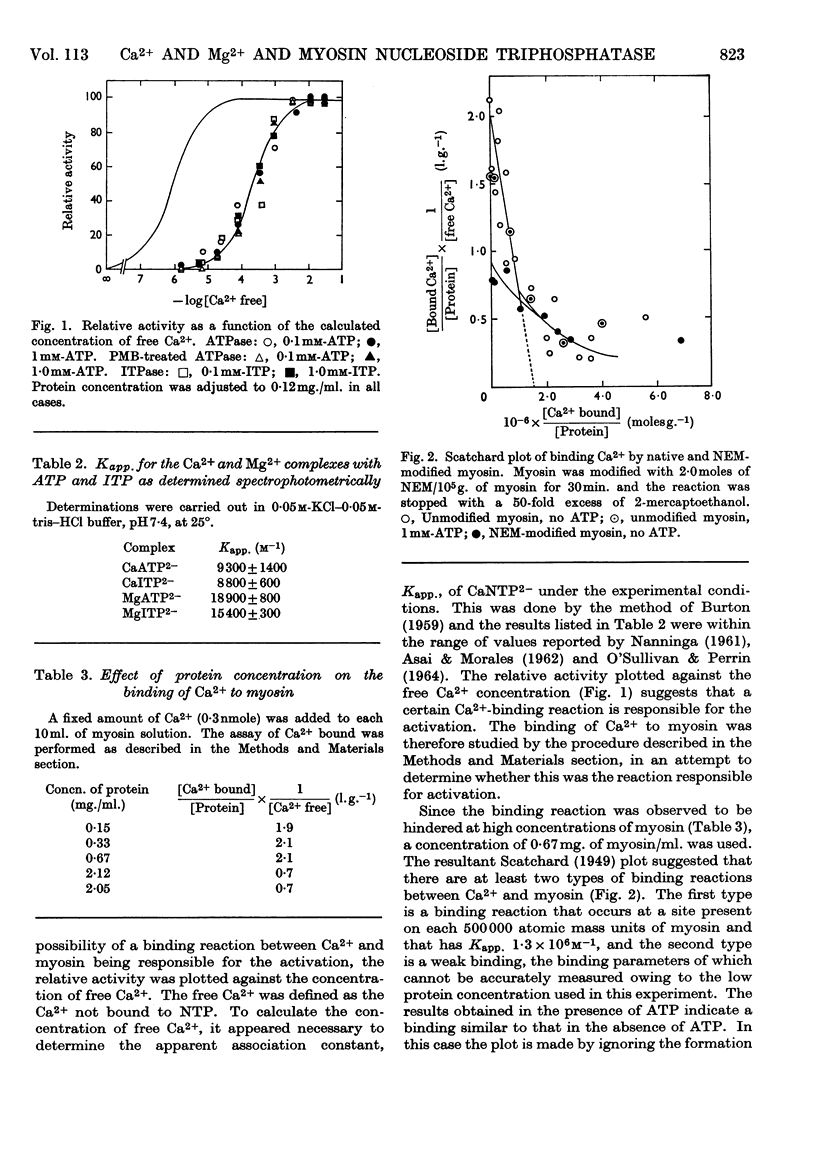
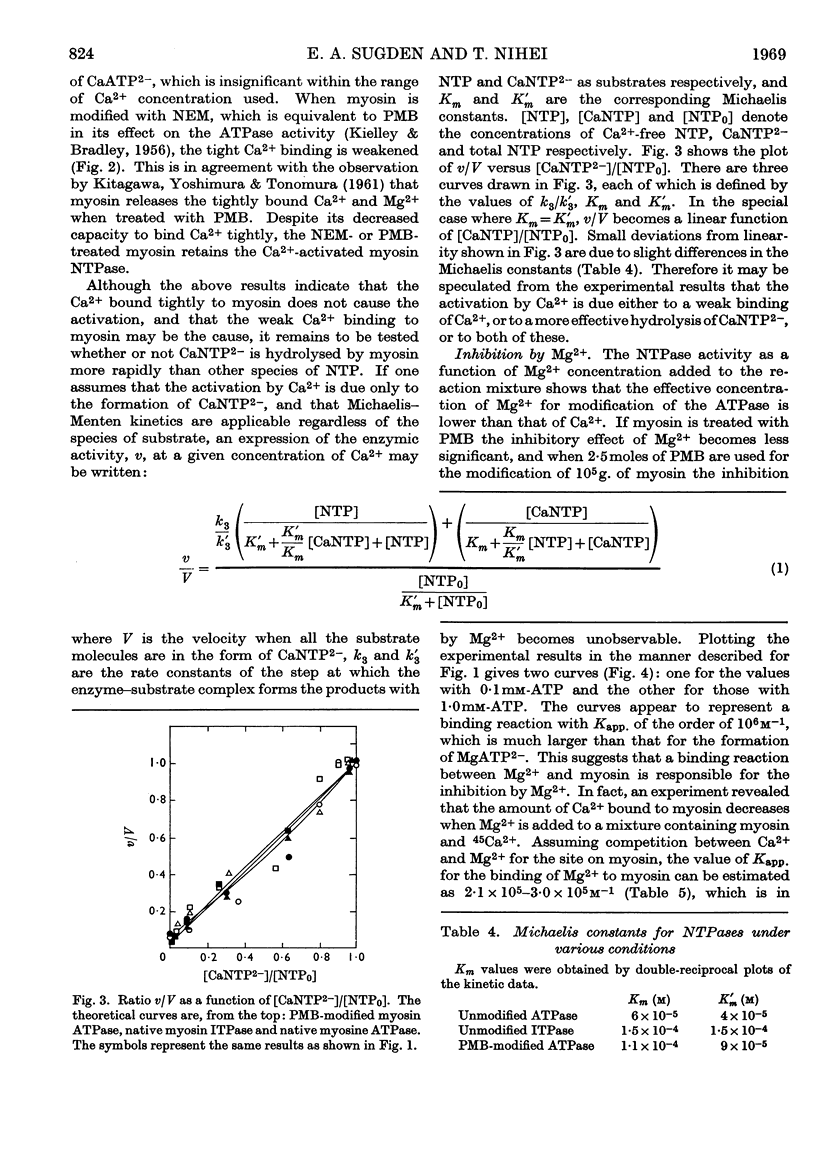
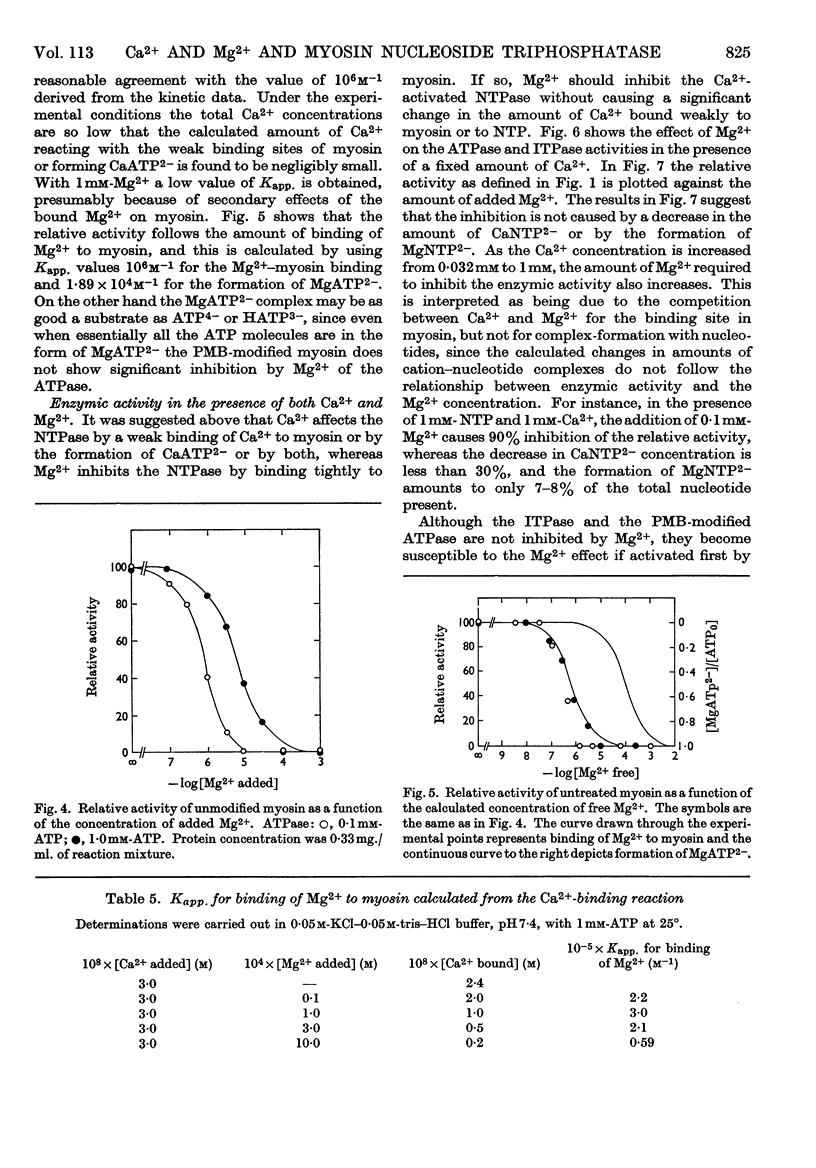
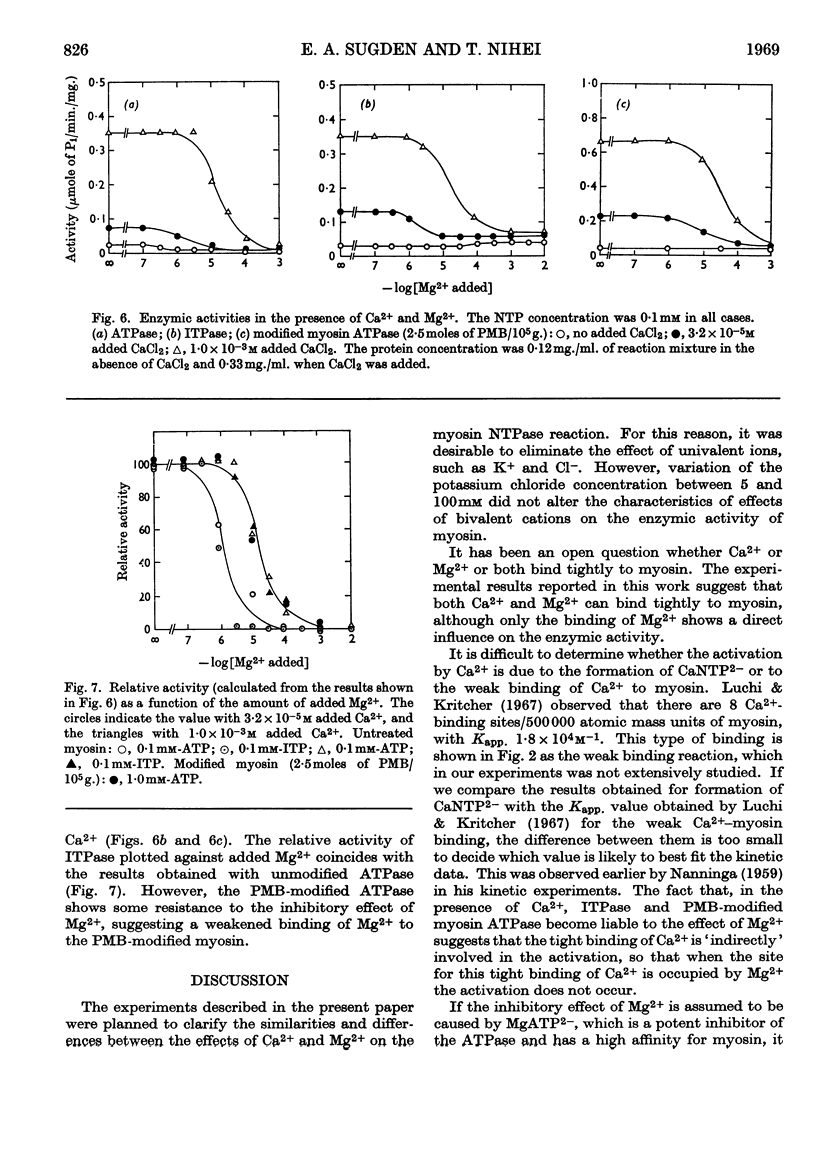
1. The effects of Ca2+ and Mg2+ on the enzymic activity of myosin were studied with myosin preparations treated by the ion-exchange resin Chelex-100. A reaction mixture containing 0·05m-potassium chloride was chosen in which the effects of univalent ions such as K+, Na+ and Cl− do not change significantly with small variations in their concentrations. 2. The relationship between the rate of hydrolysis of ATP or ITP and the concentration of Ca2+ suggests that a relatively weak binding of Ca2+ either to myosin or to the substrate nucleotide is responsible for the activation of the enzymic activity. According to the experiments with an ultrafiltration technique, the binding of Ca2+ to myosin proceeds in at least two steps, the first occurring at one site on every 500000 atomic mass units of myosin with an apparent association constant, Kapp., 1·3×106m−1, and the second seeming to be so weak that its binding parameters cannot be determined by the method used. The first type of Ca2+ binding is not observable with N-ethylmaleimide-modified myosin, yet this modified myosin shows activation by Ca2+ of its adenosine triphosphatase and inosine triphosphatase. 3. The inhibition by Mg2+ can be related to a binding reaction of Mg2+ with myosin having Kapp. ∼106m−1. Mg2+ replaces the Ca2+ bound tightly to myosin. The Kapp. for Mg2+–myosin binding calculated by assuming a competition between Ca2+ and Mg2+ for the same site is 2·1×105−3·0×105m−1. When myosin is modified with a thiol reagent (p-mercuribenzoate) at a certain ratio to myosin, the inhibition by Mg2+ becomes unobservable. 4. The behaviour of the hydrolytic activity of myosin on ATP or ITP in the presence of both Ca2+ and Mg2+ is consistent with the explanation that the inhibition by Mg2+ is due to the tight binding of Mg2+ to myosin, whereas the activation by Ca2+ is caused either by a weak binding of Ca2+ to myosin or by CaATP2− or by both.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUM J. J., FELAUER E. Effect of dinitrophenol on the interaction between myosin and nucleotides. Arch Biochem Biophys. 1959 Apr;81(2):285–299. doi: 10.1016/0003-9861(59)90206-1. [DOI] [PubMed] [Google Scholar]
- BURTON K. Formation constants for the complexes of adenosine di- or tri-phosphate with magnesium or calcium ions. Biochem J. 1959 Feb;71(2):388–395. doi: 10.1042/bj0710388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIELLEY W. W., BRADLEY L. B. The relationship between sulfhydryl groups and the activation of myosin adenosinetriphosphatase. J Biol Chem. 1956 Feb;218(2):653–659. [PubMed] [Google Scholar]
- KITAGAWA S., YOSHIMURA J., TONOMURA Y. On the active site of myosin A-adenosine triphosphatase. II. Properties of the trinitrophenyl enzyme and the enzyme free from divalent cations. J Biol Chem. 1961 Mar;236:902–906. [PubMed] [Google Scholar]
- LOWENSTEIN J. M. The stimulation of transphosphorylation by alkali-metal ions. Biochem J. 1960 May;75:269–274. doi: 10.1042/bj0750269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecocq J., Inesi G. Determination of inorganic phosphate in the presence of adenosine triphosphate by the molybdo-vanadate method. Anal Biochem. 1966 Apr;15(1):160–163. doi: 10.1016/0003-2697(66)90260-0. [DOI] [PubMed] [Google Scholar]
- Luchi R. J., Kritcher E. M. Drug effects on cardiac myosin adenosine triphosphatase activity. J Pharmacol Exp Ther. 1967 Dec;158(3):540–545. [PubMed] [Google Scholar]
- MORALES M. F., HOTTA K. The adenosine triphosphatase activity of myosin B treated with S-beta-aminoethylisothiuronium. J Biol Chem. 1960 Jul;235:1979–1986. [PubMed] [Google Scholar]
- MORI K. Direct microcomplexometric analysis of calcium in biological materials. Arch Biochem Biophys. 1959 Aug;83:552–562. doi: 10.1016/0003-9861(59)90064-5. [DOI] [PubMed] [Google Scholar]
- Mattocks P. W., Jr, Keswani G. B., Dowben R. M. Studies on myosin-azomercurial complexes. Biochemistry. 1967 Dec;6(12):3751–3756. doi: 10.1021/bi00864a019. [DOI] [PubMed] [Google Scholar]
- NANNINGA L. B. Investigation on the effect of calcium ions on the splitting of adenosinetriphosphate by myosin. Biochim Biophys Acta. 1959 Nov;36:191–202. doi: 10.1016/0006-3002(59)90084-8. [DOI] [PubMed] [Google Scholar]
- NANNINGA L. B. The association constant of the complexes of adenosine triphosphate with magnesium, calcium, strontium, and barium ions. Biochim Biophys Acta. 1961 Dec 9;54:330–338. doi: 10.1016/0006-3002(61)90373-0. [DOI] [PubMed] [Google Scholar]
- Nihei T. Adenosine triphosphatase activated by magnesium and superprecipitation of myosin B. Nature. 1967 Aug 19;215(5103):886–887. doi: 10.1038/215886a0. [DOI] [PubMed] [Google Scholar]
- OFFER G. W. THE ANTAGONISTIC ACTION OF MAGNESIUM IONS AND ETHYLENEDIAMINETETRAACETATE ON MYOSIN A ATPASE (POTASSIUM ACTIVATED). Biochim Biophys Acta. 1964 Sep 18;89:566–569. doi: 10.1016/0926-6569(64)90090-2. [DOI] [PubMed] [Google Scholar]
- PERRY S. V., COTTERILL J. THE ACTION OF THIOL REAGENTS ON THE ADENOSINE-TRIPHOSPHATASE ACTIVITIES OF HEAVY MEROMYOSIN AND L-MYOSIN. Biochem J. 1965 Jul;96:224–230. doi: 10.1042/bj0960224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreter F. A., Seidel J. C., Gergely J. Studies on myosin from red and white skeletal muscles of the rabbit. I. Adenosine triphosphatase activity. J Biol Chem. 1966 Dec 25;241(24):5772–5776. [PubMed] [Google Scholar]
- Tonomura Y., Appel P., Morales M. On the molecular weight of myosin. II. Biochemistry. 1966 Feb;5(2):515–521. doi: 10.1021/bi00866a017. [DOI] [PubMed] [Google Scholar]
- Warren J. C., Stowring L., Morales M. F. The effect of structure-disrupting ions on the activity of myosin and other enzymes. J Biol Chem. 1966 Jan 25;241(2):309–316. [PubMed] [Google Scholar]


