Abstract
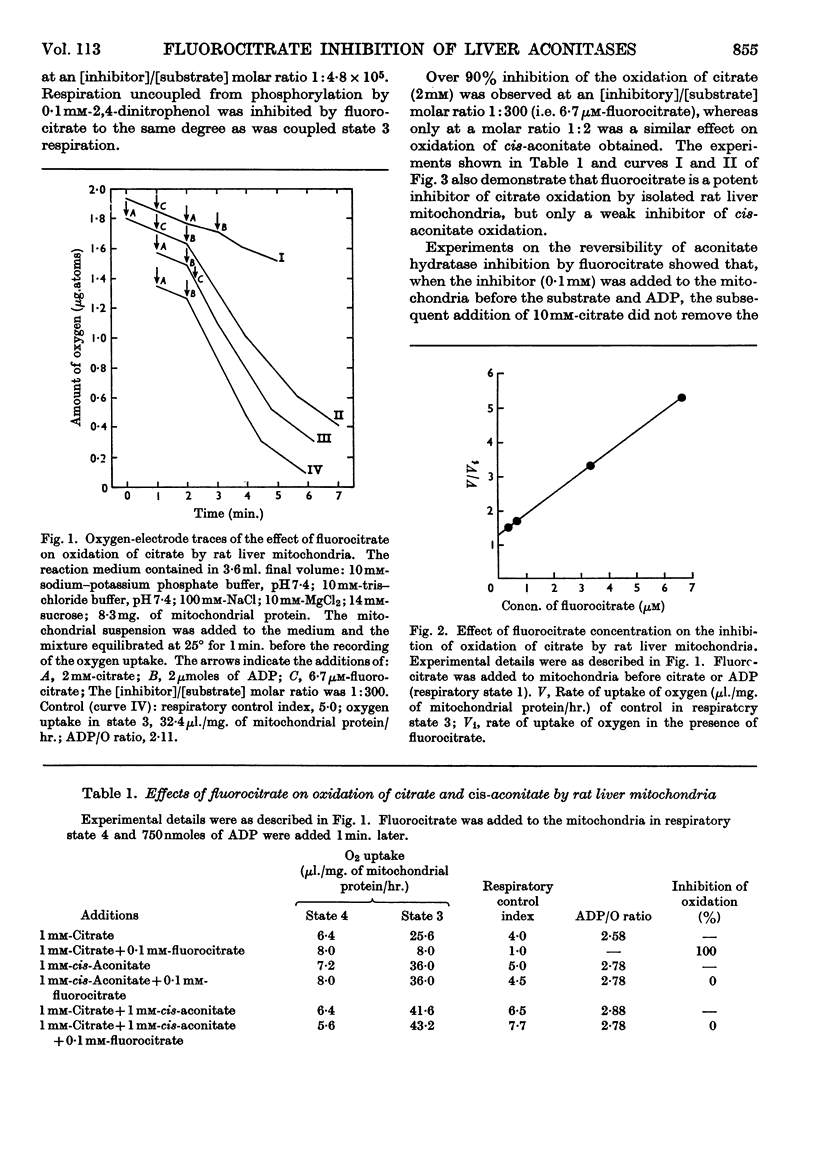
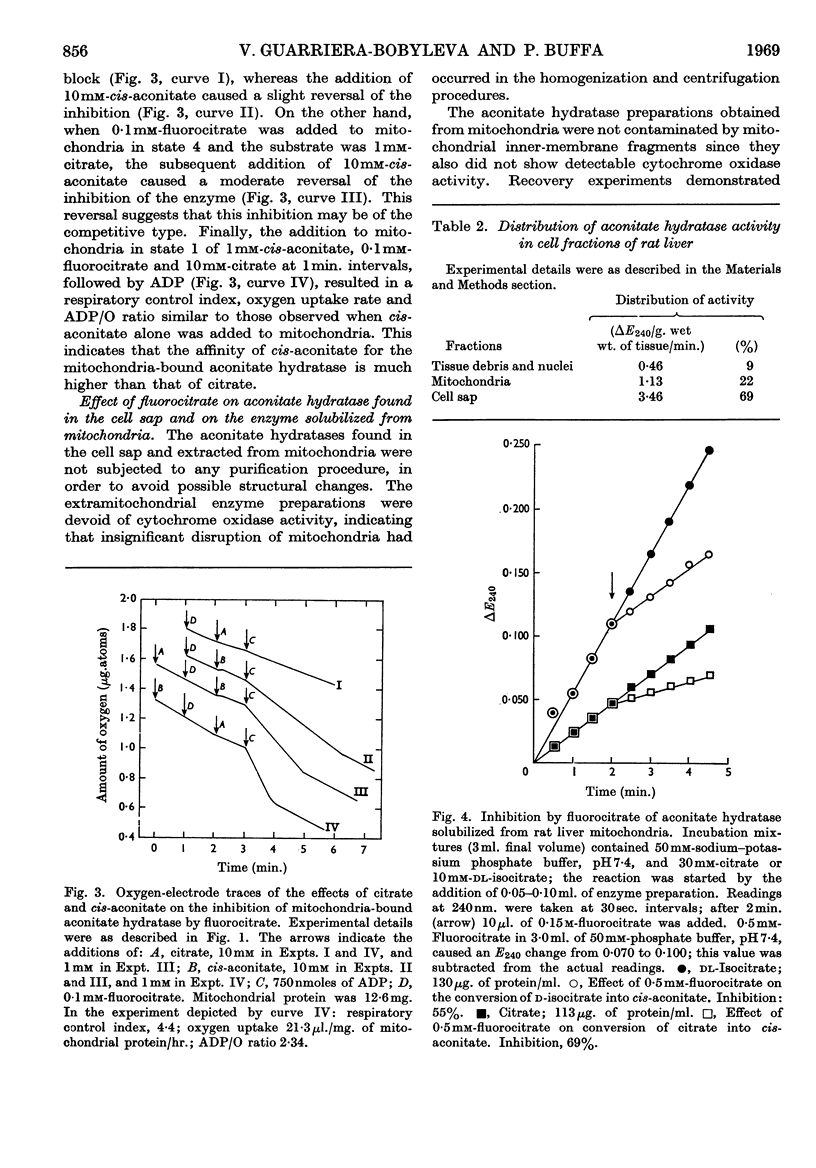
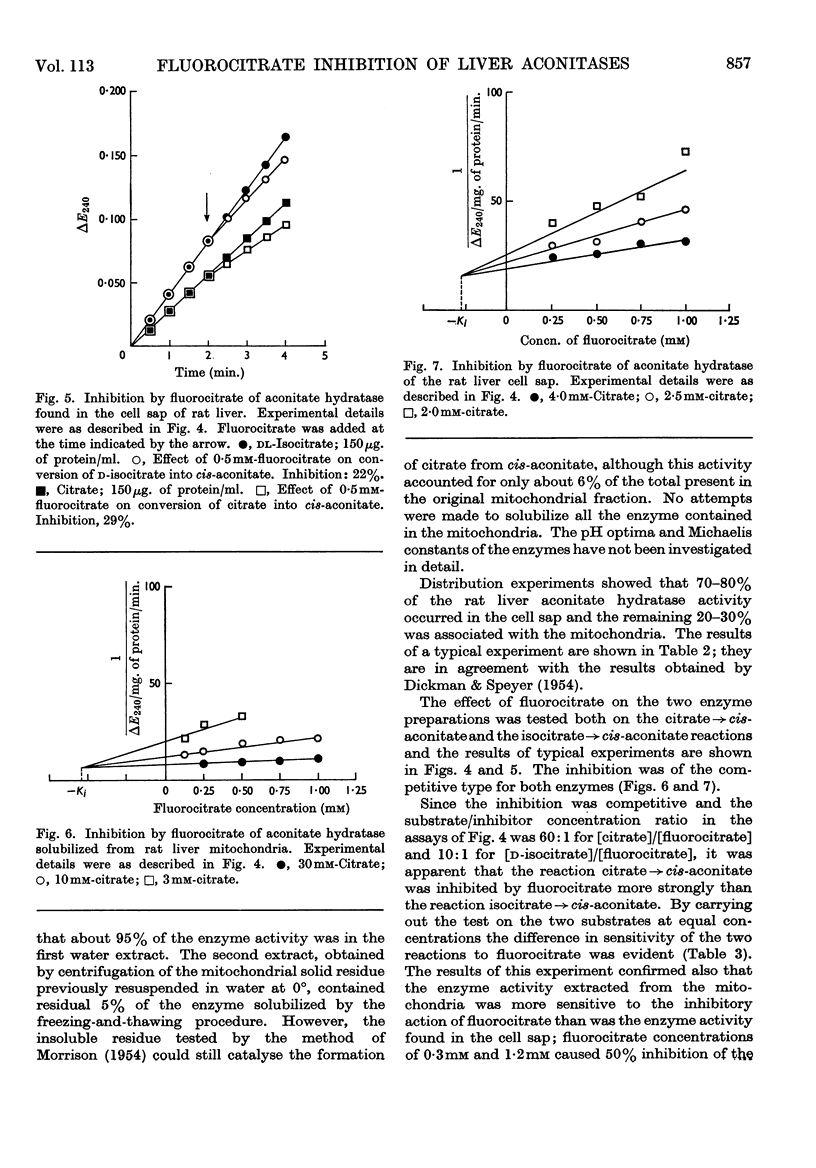
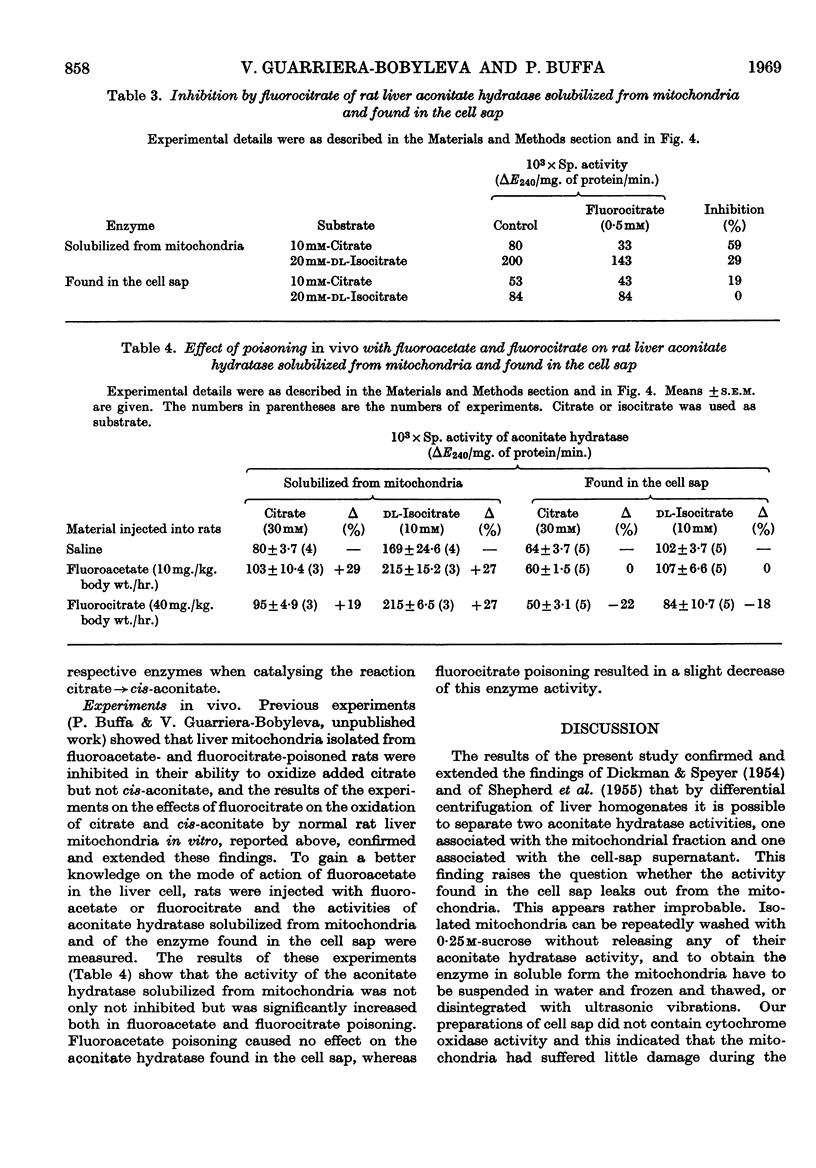
1. The effects of synthetic fluorocitrate were studied on: (a) the oxidation of citrate and cis-aconitate by rat liver mitochondria; (b) the activity of the aconitate hydratase found in the liver cell sap; (c) the activity of the aconitate hydratase solubilized from liver mitochondria. 2. Fluorocitrate was found to be a potent inhibitor of oxidation of citrate but only a weak inhibitor of oxidation of cis-aconitate: 6·7μm-fluorocitrate (containing 4% of the inhibitory isomer) caused 94% inhibition of the oxidation of citrate (2mm) whereas 1·0mm-fluorocitrate was necessary to provoke the same inhibition when cis-aconitate (2mm) was the substrate. The degree of inhibition varied in relation to the respiratory state of mitochondria when fluorocitrate was added. The inhibition could be partially reversed by cis-aconitate. 3. The aconitate hydratase extracted from the mitochondria was much less inhibited by fluorocitrate than was the mitochondria-bound enzyme, and the aconitate hydratase found in the cell sap was even less sensitive. 0·3mm-Fluorocitrate was required to cause 50% inhibition of the reaction citrate→cis-aconitate, catalysed by the aconitate hydratase extracted from the mitochondria, and 1·2m-fluorocitrate for the extramitochondrial enzyme. For both enzymes the reaction citrate→cis-aconitate was 2–3 times more sensitive to fluorocitrate than was the reaction isocitrate→cis-aconitate. The inhibition was of the competitive type for both reactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUFFA P., PETERS R. A. The in vivo formation of citrate induced by fluoroacetate and its significance. J Physiol. 1949 Dec;110(3-4):488–500. doi: 10.1113/jphysiol.1949.sp004456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSCH H., POTTER V. R. Localization of the enzymatic block in kidneys of rats treated with fluoroacetate. Proc Soc Exp Biol Med. 1952 Oct;81(1):172–175. doi: 10.3181/00379727-81-19814. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- LOTSPEICH W. D., PETERS R. A., WILSON T. H. The inhibition of aconitase by 'Inhibitor fractions' isolated from tissues poisoned with fluoroacetate. Biochem J. 1952 Apr;51(1):20–25. doi: 10.1042/bj0510020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON J. F., PETERS R. A. Biochemistry of fluoroacetate poisoning: the effect of fluorocitrate on purified aconitase. Biochem J. 1954 Nov;58(3):473–479. doi: 10.1042/bj0580473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON J. F. The purification of aconitase. Biochem J. 1954 Jan;56(1):99–105. doi: 10.1042/bj0560099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS R. A. Further experiments on the inhibition of aconitase by enzymically prepared fluorocitric acid. Biochem J. 1961 May;79:261–268. doi: 10.1042/bj0790261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS R. A. Lethal synthesis. Proc R Soc Lond B Biol Sci. 1952 Feb 28;139(895):143–170. doi: 10.1098/rspb.1952.0001. [DOI] [PubMed] [Google Scholar]
- Peters R. A., Shorthouse M. Aconitase, a complex with two active centres. Nature. 1969 Feb 22;221(5182):774–775. doi: 10.1038/221774a0. [DOI] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- SHEPHERD J. A., LI Y. W., MASON E. E., ZIFFREN S. E. The distribution of aconitase and fumarase in homogenates of human liver. J Biol Chem. 1955 Mar;213(1):405–408. [PubMed] [Google Scholar]
- WARD P. F., PETERS R. A. The chemical and biochemical properties of fluorocitric acid. Biochem J. 1961 Mar;78:661–668. doi: 10.1042/bj0780661. [DOI] [PMC free article] [PubMed] [Google Scholar]


