Abstract
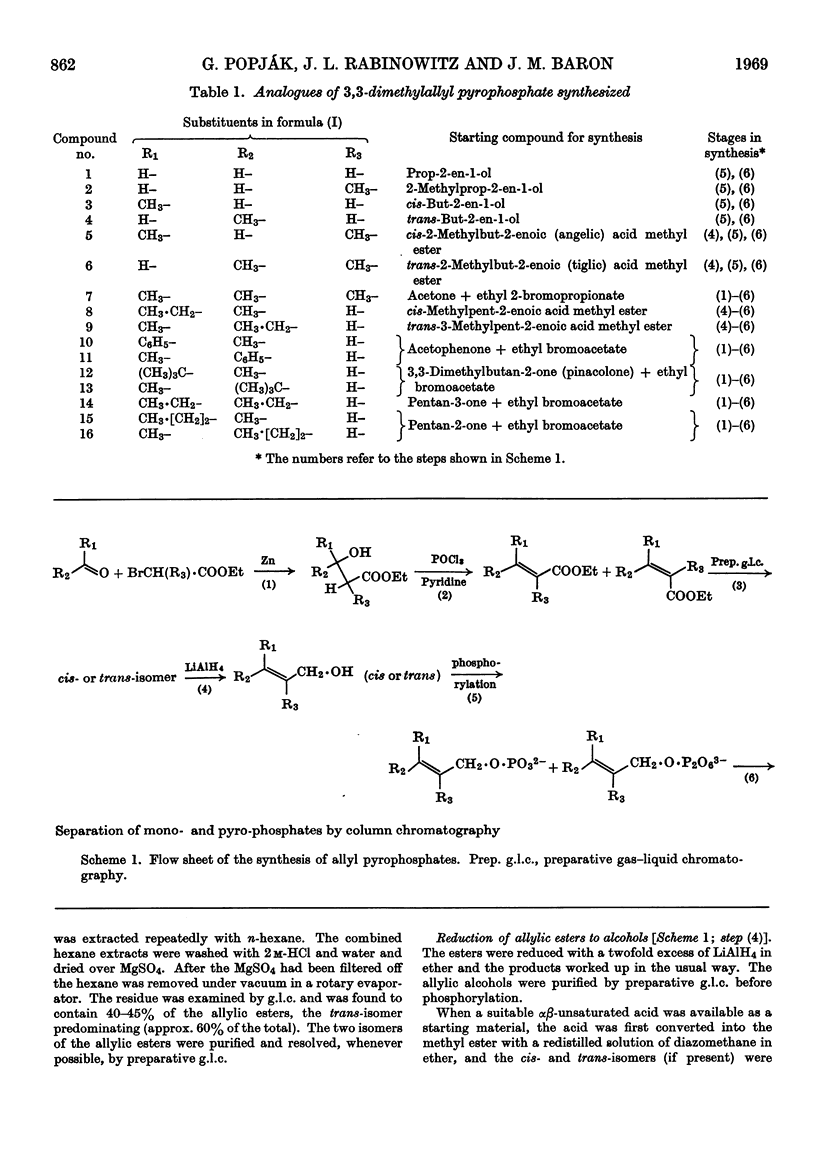
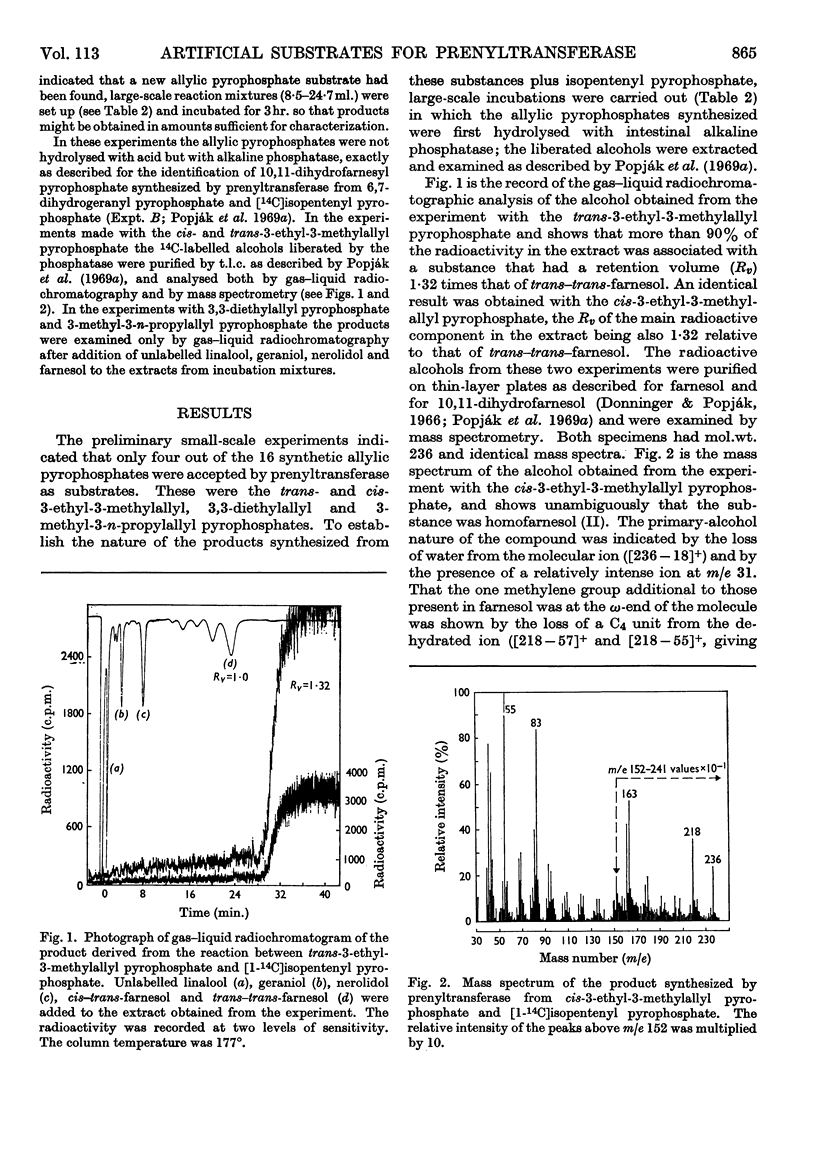
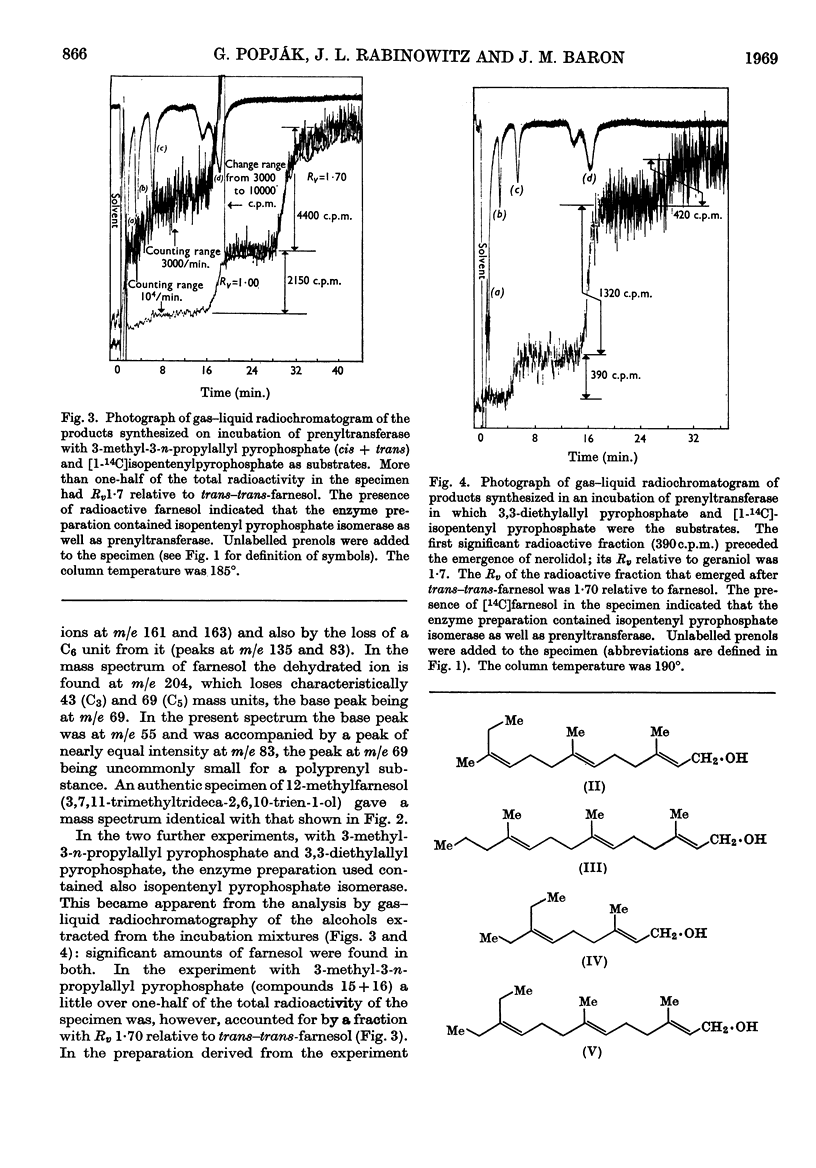
Four out of 16 new allylic pyrophosphates synthesized were found to be artificial substrates for liver prenyltransferase (EC 2.5.1.1). These were the trans-and the cis-3-ethyl-3-methylallyl, the 3,3-diethylallyl and the (mixture of cis and trans) 3-methyl-3-n-propylallyl pyrophosphates. The products synthesized from these substrates and isopentenyl pyrophosphate were the appropriate homo- and bishomo-farnesyl pyrophosphates. Substitution of 3,3-dimethylallyl pyrophosphate at C-2 with a methyl group destroyed its reactivity with the enzyme. Neither the unsubstituted allyl pyrophosphate nor the cis- or trans-3-methylallyl pyrophosphate could be condensed with isopentenyl pyrophosphate. Thus the simplest allylic substrate for prenyltransferase is 3,3-dimethylallyl pyrophosphate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornforth J. W., Cornforth R. H., Popják G., Yengoyan L. Studies on the biosynthesis of cholesterol. XX. Steric course of decarboxylation of 5-pyrophosphomevalonate and of the carbon to carbon bond formation in the biosynthesis of farnesyl pyrophosphate. J Biol Chem. 1966 Sep 10;241(17):3970–3987. [PubMed] [Google Scholar]
- Donninger C., Popják G. Studies on the biosynthesis of cholesterol. 18. The stereospecificity of mevaldate reductase and the biosynthesis of asymmetrically labelled farnesyl pyrophosphate. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):465–491. doi: 10.1098/rspb.1966.0003. [DOI] [PubMed] [Google Scholar]
- Holloway P. W., Popják G. The purification of 3,3-dimethylallyl- and geranyl-transferase and of isopentenyl pyrophosphate isomerase from pig liver. Biochem J. 1967 Jul;104(1):57–70. doi: 10.1042/bj1040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPJAK G., CORNFORTH J. W., CORNFORTH R. H., RYHAGE R., GOODMAN D. S. Studies on the biosynthesis of cholesterol. XVI. Chemical synthesis of 1-H2-3-2-C-14- and 1-D2-2-C-14-trans-trans-farnesyl pyrophosphate and their utilization in squalene biosynthesis. J Biol Chem. 1962 Jan;237:56–61. [PubMed] [Google Scholar]
- Popják G., Holloway P. W., Baron J. M. Synthesis of 10,11-dihydrofarnesyl pyrophosphate from 6,7-dihydrogeranyl pyrophosphate by prenyltransferase. Biochem J. 1969 Feb;111(3):325–332. doi: 10.1042/bj1110325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popják G., Holloway P. W., Bond R. P., Roberts M. Analogues of geranyl pyrophosphate as inhibitors of prenyltransferase. Biochem J. 1969 Feb;111(3):333–343. doi: 10.1042/bj1110333. [DOI] [PMC free article] [PubMed] [Google Scholar]


