Abstract
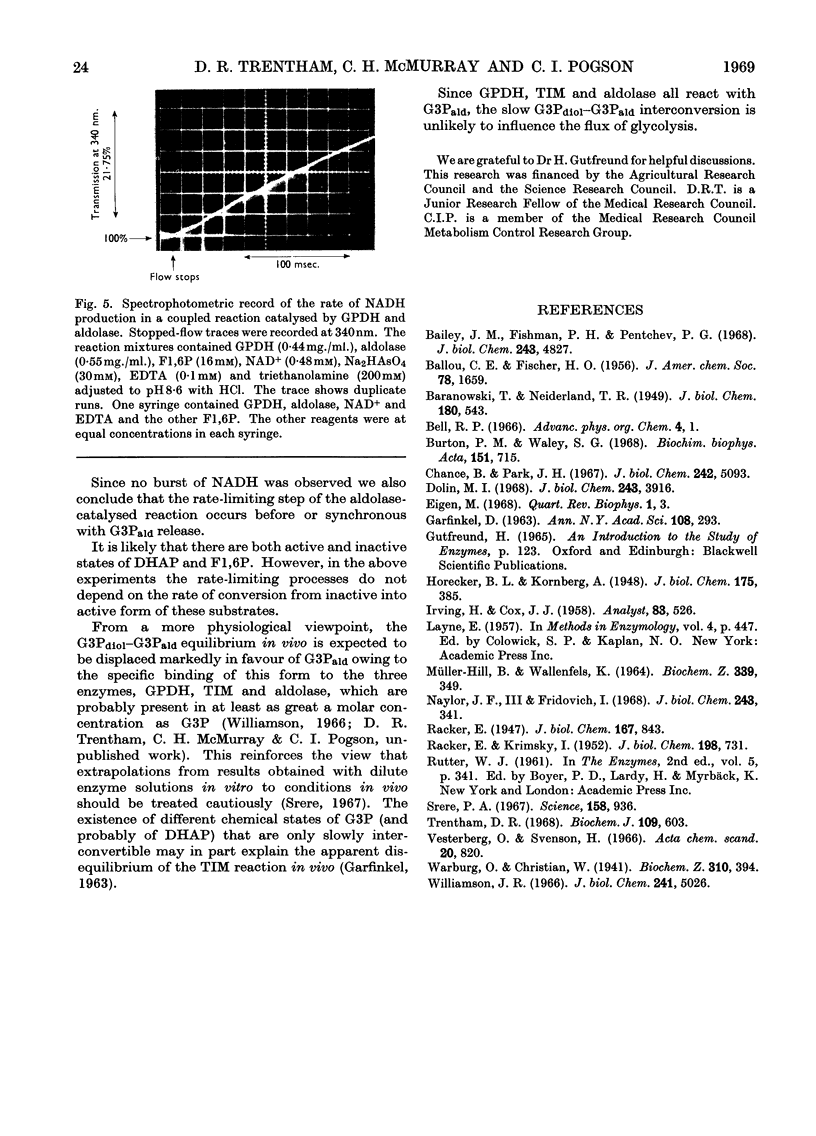
Glyceraldehyde 3-phosphate exists as the geminal diol and the free aldehyde in the molar ratio 29:1 in aqueous solution. The rate constant of the conversion of diol into aldehyde is 8·7×10−2sec.−1 in the pH range 7·3–8·6 at 20°. The free aldehyde is the substrate for d-glyceraldehyde 3-phosphate dehydrogenase. Over a wide concentration range of enzyme the rate of conversion of diol into aldehyde is the rate-limiting process in the catalytic oxidation of d-glyceraldehyde 3-phosphate by NAD+. Aldolase and triose phosphate isomerase both liberate d-glyceraldehyde 3-phosphate as the aldehyde. This suggests that the relatively slow diol–aldehyde interconversion does not restrict the rate of glycolysis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Fishman P. H., Pentchev P. G. Studies on mutarotases. II. Investigations of possible rate-limiting anomerizations in glucose metabolism. J Biol Chem. 1968 Sep 25;243(18):4827–4831. [PubMed] [Google Scholar]
- Burton P. M., Waley S. G. Kinetics of triose phosphate isomerase. Biochim Biophys Acta. 1968 Mar 25;151(3):714–715. doi: 10.1016/0005-2744(68)90028-4. [DOI] [PubMed] [Google Scholar]
- Chance B., Park J. H. The properties and enzymatic significance of the enzyme-diphosphopyridine nucleotide compound of 3-phosphoglyceraldehyde dehydrogenase. J Biol Chem. 1967 Nov 10;242(21):5093–5105. [PubMed] [Google Scholar]
- Dolin M. I. Kinetics of malic-lactic transhydrogenase. Effect of the keto-enol tautomerism of oxalacetate on the kinetics of oxalacetate formation and utilization. J Biol Chem. 1968 Jul 25;243(14):3916–3923. [PubMed] [Google Scholar]
- GARFINKEL D. Digital computer simulation of systems apparently compartmented at the cellular level. Ann N Y Acad Sci. 1963 May 10;108:293–304. doi: 10.1111/j.1749-6632.1963.tb13381.x. [DOI] [PubMed] [Google Scholar]
- Naylor J. F., 3rd, Fridovich I. The form of the aldehyde susceptible to enzymic oxidation. J Biol Chem. 1968 Jan 25;243(2):341–345. [PubMed] [Google Scholar]
- RACKER E., KRIMSKY I. The mechanism of oxidation of aldehydes by glyceralde-hyde-3-phosphate dehydrogenase. J Biol Chem. 1952 Oct;198(2):731–743. [PubMed] [Google Scholar]
- Srere P. A. Enzyme concentrations in tissues. Science. 1967 Nov 17;158(3803):936–937. doi: 10.1126/science.158.3803.936. [DOI] [PubMed] [Google Scholar]
- Trentham D. R. Aspects of the chemistry of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1968 Oct;109(4):603–612. doi: 10.1042/bj1090603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J Biol Chem. 1966 Nov 10;241(21):5026–5036. [PubMed] [Google Scholar]