Abstract
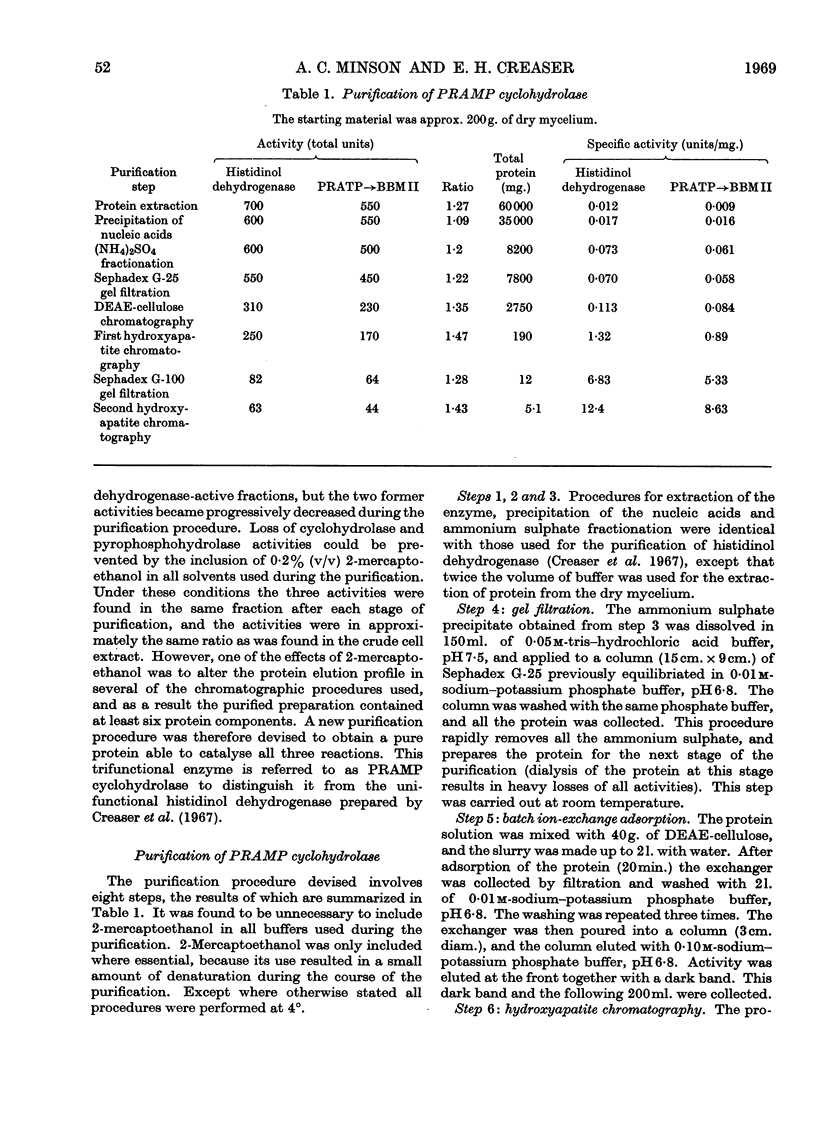
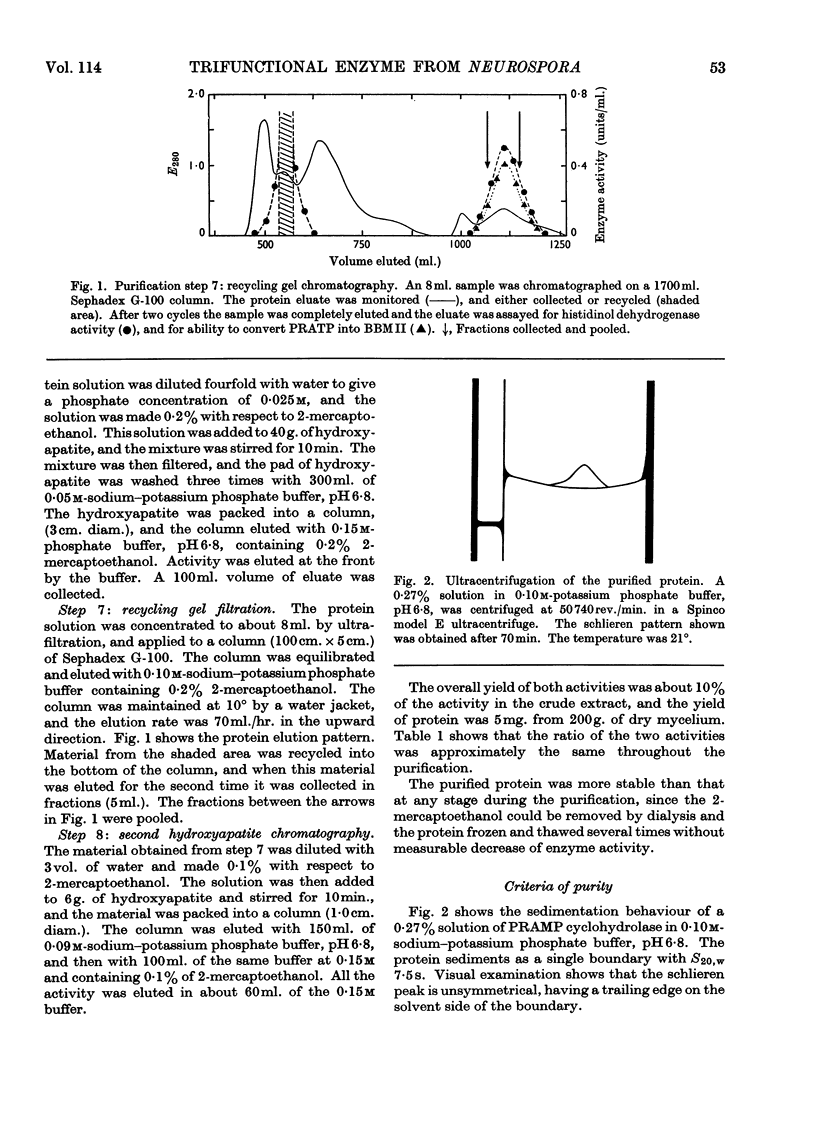
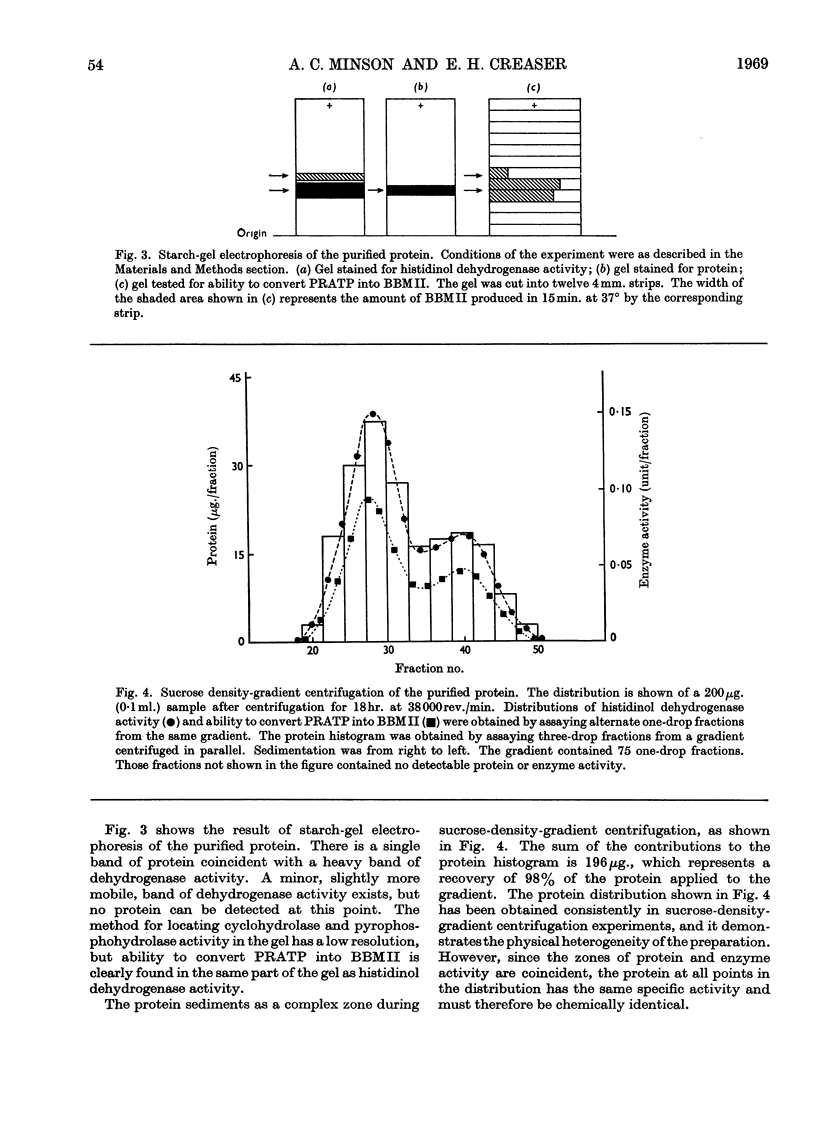
1. A procedure is described for the purification of an enzyme from Neurospora crassa that has three catalytic functions. These are 1-N-(5′-phosphoribosyl)-ATP pyrophosphohydrolase, 1-N-(5′-phosphoribosyl)-AMP cyclohydrolase and histidinol dehydrogenase (l-histidinol–NAD oxidoreductase, EC 1.1.1.23), and are responsible for the catalysis of reactions 2, 3 and 10 in the histidine pathway. The ratio of these three catalytic activities remains approximately the same throughout the purification procedure. Evidence is presented that the purified preparations contain a single protein exhibiting association–dissociation equilibria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMED A., CASE M. E., GILES N. H. THE NATURE OF COMPLEMENTATION AMONG MUTANTS IN THE HISTIDINE-3 REGION OF NEUROSPORA CRASSA. Brookhaven Symp Biol. 1964 Dec;17:53–65. [PubMed] [Google Scholar]
- AMES B. N., GARRY B., HERZENBERG L. A. The genetic control of the enzymes of histidine biosynthesis in Salmonella typhimurium. J Gen Microbiol. 1960 Apr;22:369–378. doi: 10.1099/00221287-22-2-369. [DOI] [PubMed] [Google Scholar]
- AMES B. N., HORECKER B. L. The biosynthesis of histidine: imidazoleacetol phosphate transaminase. J Biol Chem. 1956 May;220(1):113–128. [PubMed] [Google Scholar]
- Ahmed A. Organization of the histidine-3 region of Neurospora. Mol Gen Genet. 1968;103(2):185–193. doi: 10.1007/BF00427145. [DOI] [PubMed] [Google Scholar]
- Bennett D. J., Creaser E. H. Amino acid substitutions in mutant forms of histidinol dehydrogenase from Neurospora crassa. Biochem J. 1967 Oct;105(1):39–44. doi: 10.1042/bj1050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyn M. B. Gene-enzyme relationships in histidine biosynthesis in Aspergillus nidulans. Genetics. 1967 Nov;57(3):561–570. doi: 10.1093/genetics/57.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creaser E. H., Bennett D. J., Drysdale R. B. Studies on biosynthetic enzymes. I. Mutant forms of histidinol dehydrogenase from Neurospora crassa. Can J Biochem. 1965 Jul;43(7):993–1000. doi: 10.1139/o65-112. [DOI] [PubMed] [Google Scholar]
- Creaser E. H., Bennett D. J., Drysdale R. B. The purification and properties of histidinol dehydrogenase from Neurospora crassa. Biochem J. 1967 Apr;103(1):36–41. doi: 10.1042/bj1030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R. A cluster of genes controlling three enzymes in histidine biosynthesis in Saccharomyces cerevisiae. Genetics. 1966 Mar;53(3):445–459. doi: 10.1093/genetics/53.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R. A cluster of genes controlling three enzymes in histidine biosynthesis in Saccharomyces cerevisiae. Genetics. 1966 Mar;53(3):445–459. doi: 10.1093/genetics/53.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- SMITH D. W., AMES B. N. INTERMEDIATES IN THE EARLY STEPS OF HISTIDINE BIOSYNTHESIS. J Biol Chem. 1964 Jun;239:1848–1855. [PubMed] [Google Scholar]
- SMITH D. W., AMES B. N. PHOSPHORIBOSYLADENOSINE MONOPHOSPHATE, AN INTERMEDIATE IN HISTIDINE BIOSYNTHESIS. J Biol Chem. 1965 Jul;240:3056–3063. [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WHITFIELD H. J., Jr, SMITH D. W., MARTIN R. G. SEDIMENTATION PROPERTIES OF THE ENZYMES OF THE HISTIDINE OPERON. J Biol Chem. 1964 Oct;239:3288–3291. [PubMed] [Google Scholar]


