Abstract
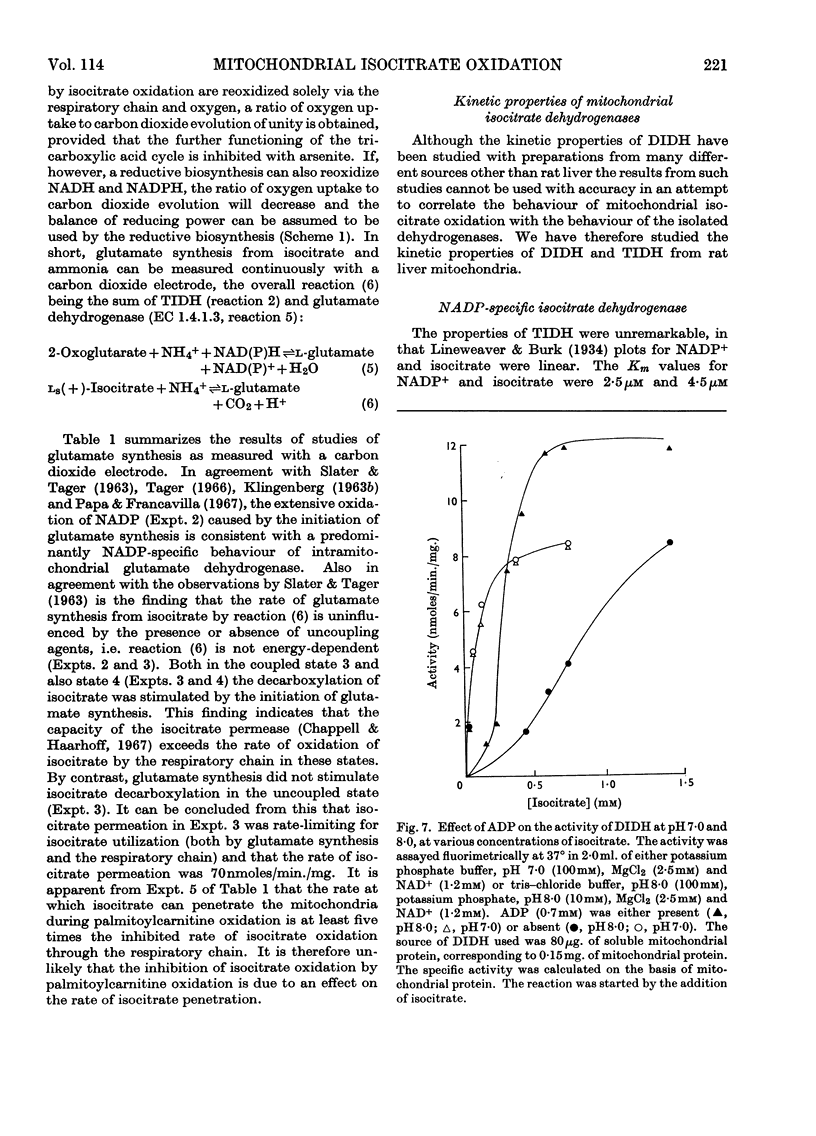
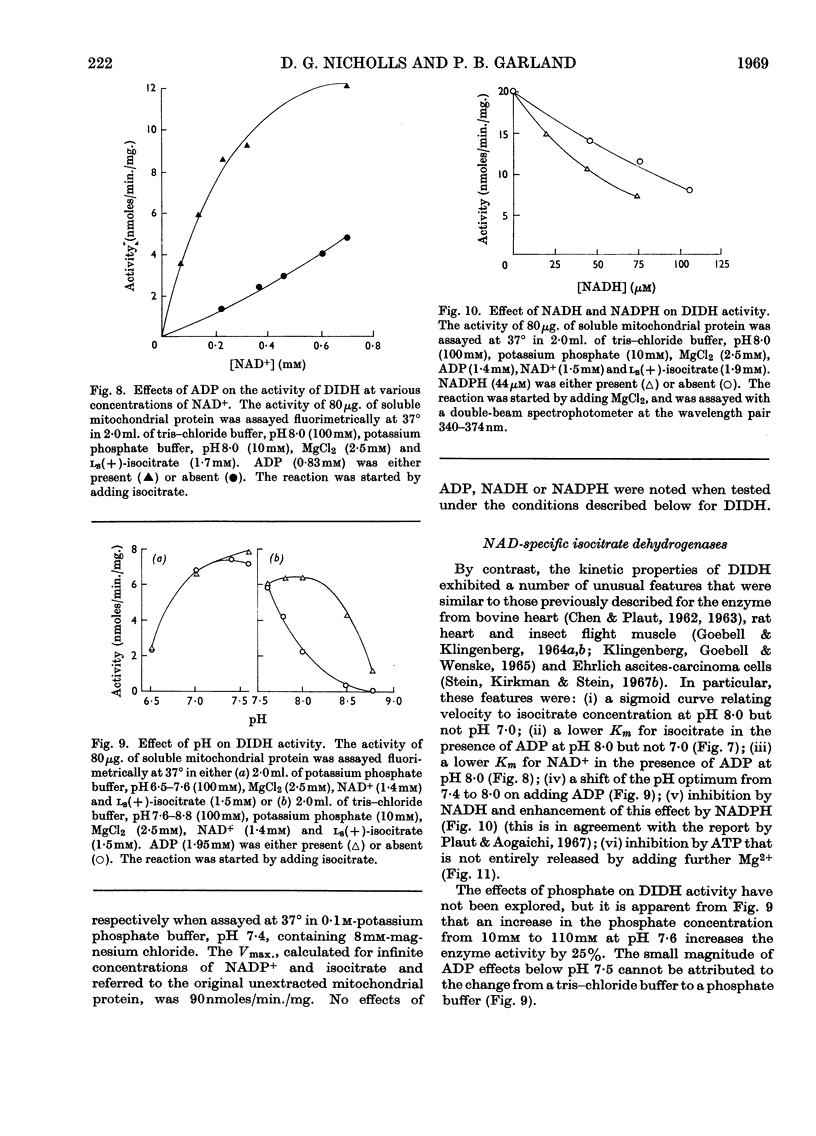
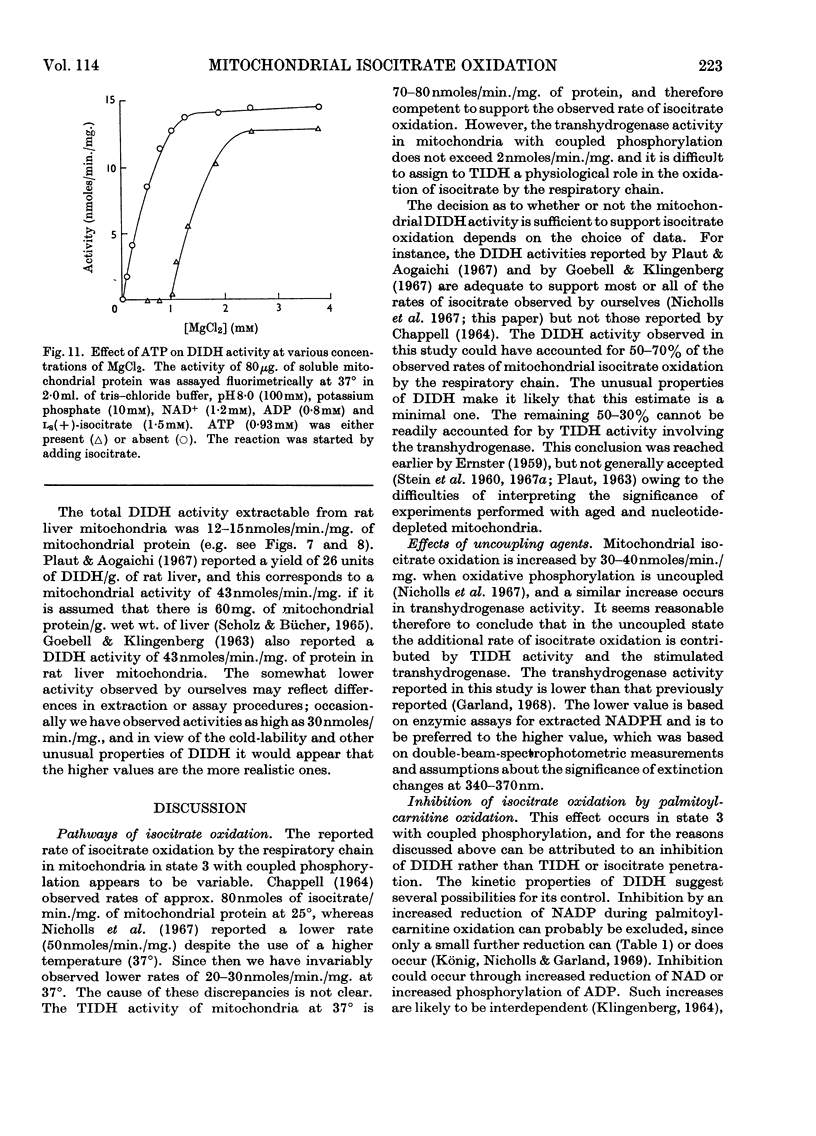
1. The factors capable of affecting the rate of isocitrate oxidation in intact mitochondria include the rate of isocitrate penetration, the activity of the NAD-specific and NADP-specific isocitrate dehydrogenases, the activity of the transhydrogenase acting from NADPH to NAD+, the rate of NADPH oxidation by the reductive synthesis of glutamate and the activity of the respiratory chain. A quantitative assessment of these factors was made in intact mitochondria. 2. The kinetic properties of the NAD-specific and NADP-specific isocitrate dehydrogenases extracted from rat liver mitochondria were examined. 3. The rate of isocitrate oxidation through the respiratory chain in mitochondria with coupled phosphorylation is approximately equal to the maximal of the NAD-specific isocitrate dehydrogenase but at least ten times as great as the transhydrogenase activity from NADPH to NAD+. 4. It is concluded that the energy-dependent inhibition of isocitrate oxidation by palmitoylcarnitine oxidation is due to an inhibition of the NAD-specific isocitrate dehydrogenase. 5. Kinetic studies of NAD-specific isocitrate dehydrogenase demonstrated that its activity could be inhibited by one or more of the following: an increased reduction of mitochondrial NAD, an increased phosphorylation of mitochondrial adenine nucleotides or a fall in the mitochondrial isocitrate concentration. 6. Uncoupling agents stimulate isocitrate oxidation by an extent equal to the associated stimulation of transhydrogenation from NADPH to NAD+. 7. A technique is described for continuously measuring with a carbon dioxide electrode the synthesis of glutamate from isocitrate and ammonia.
Full text
PDF

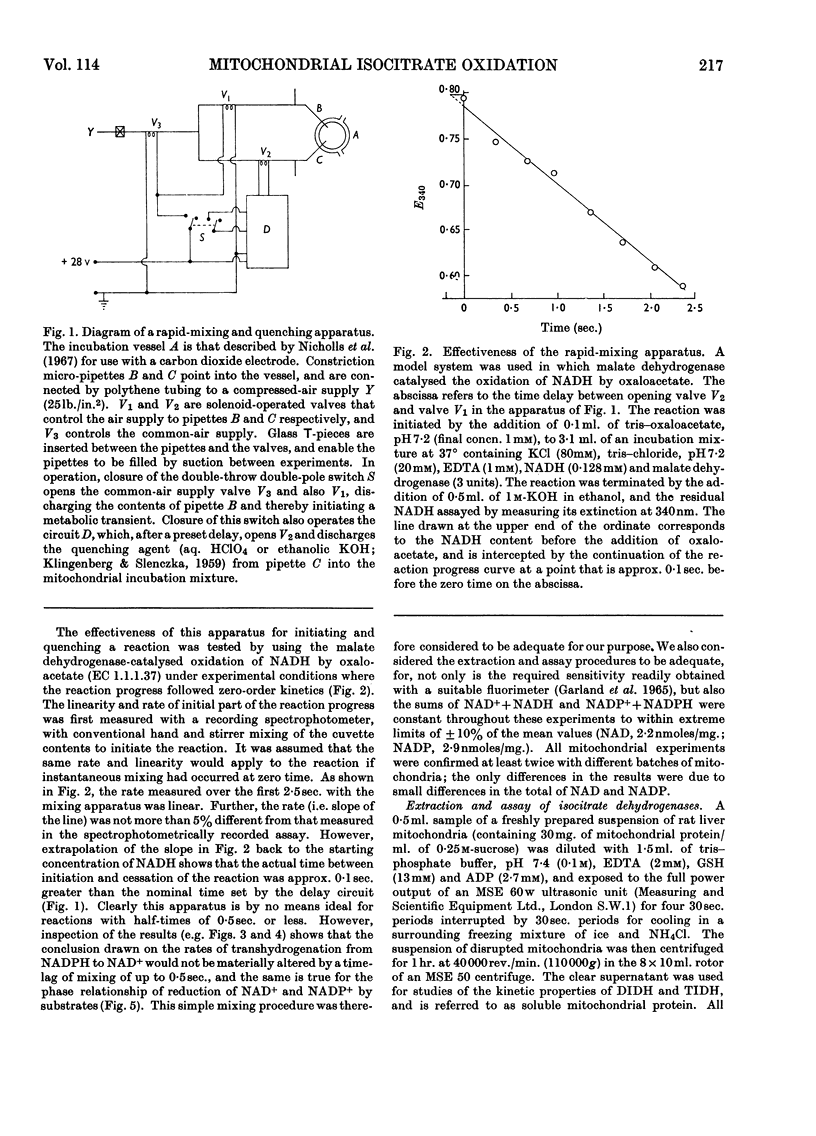
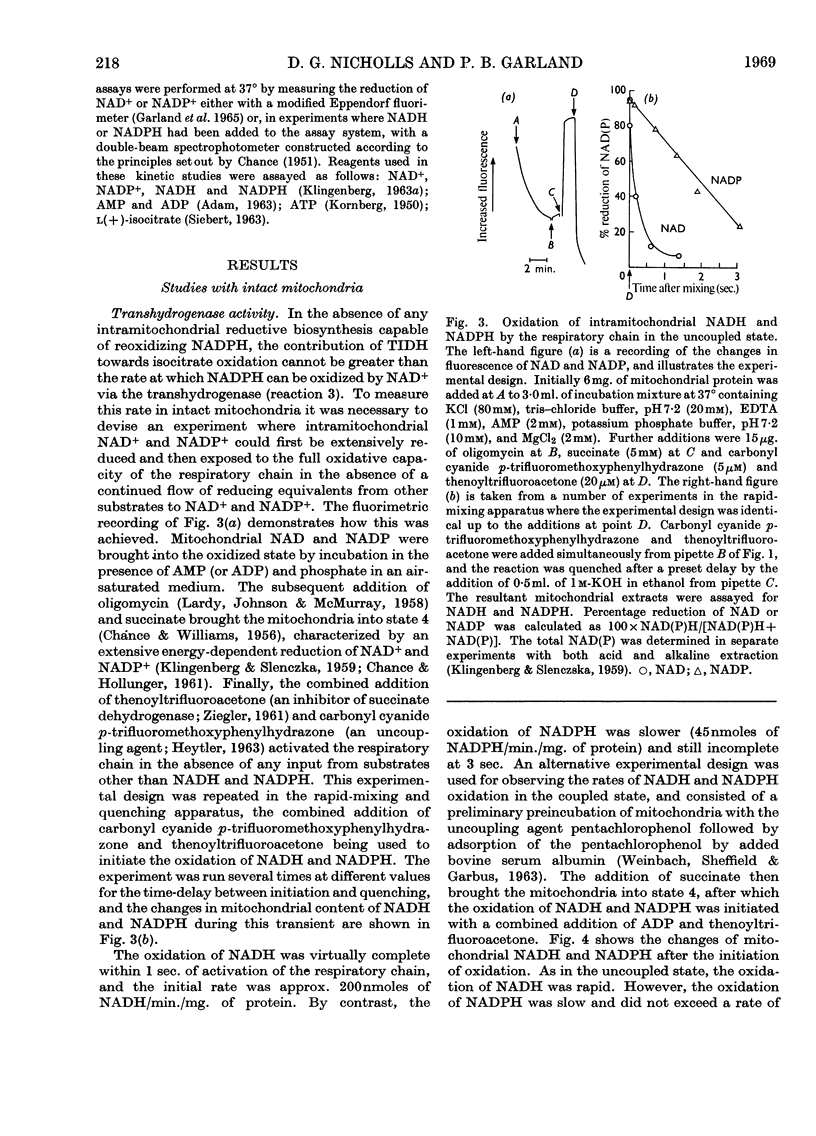
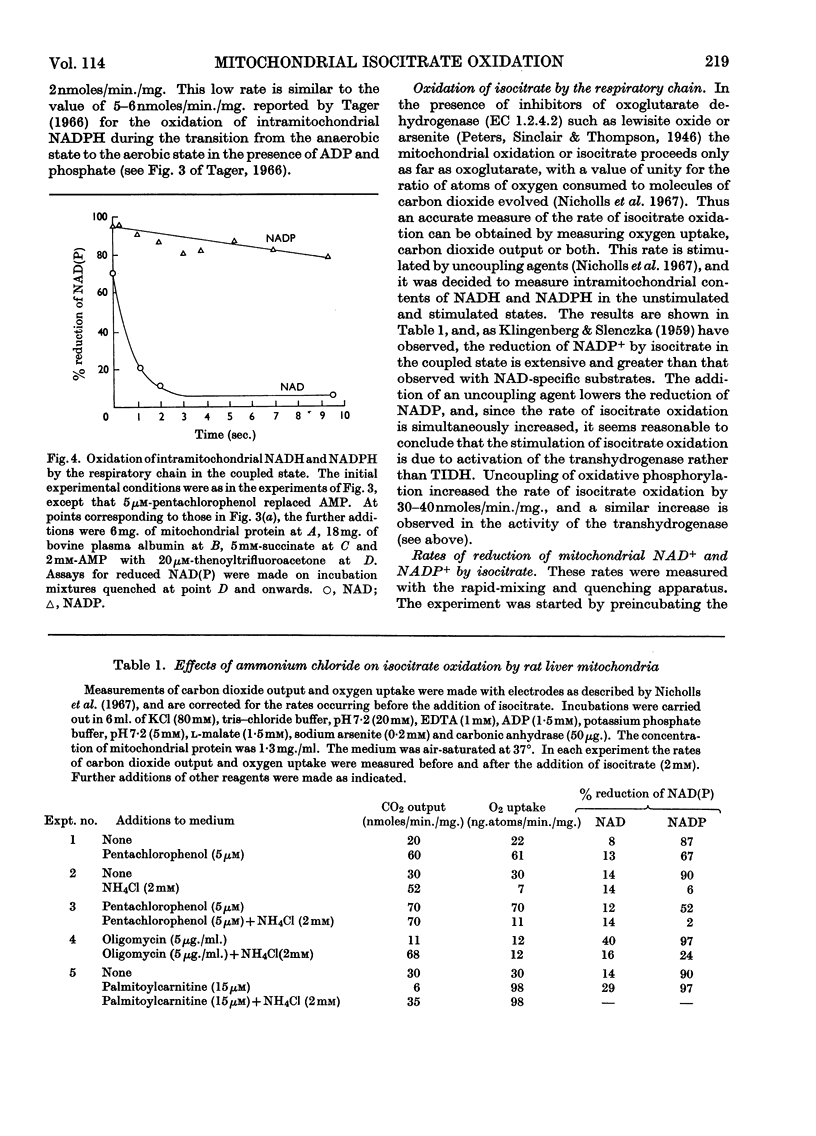
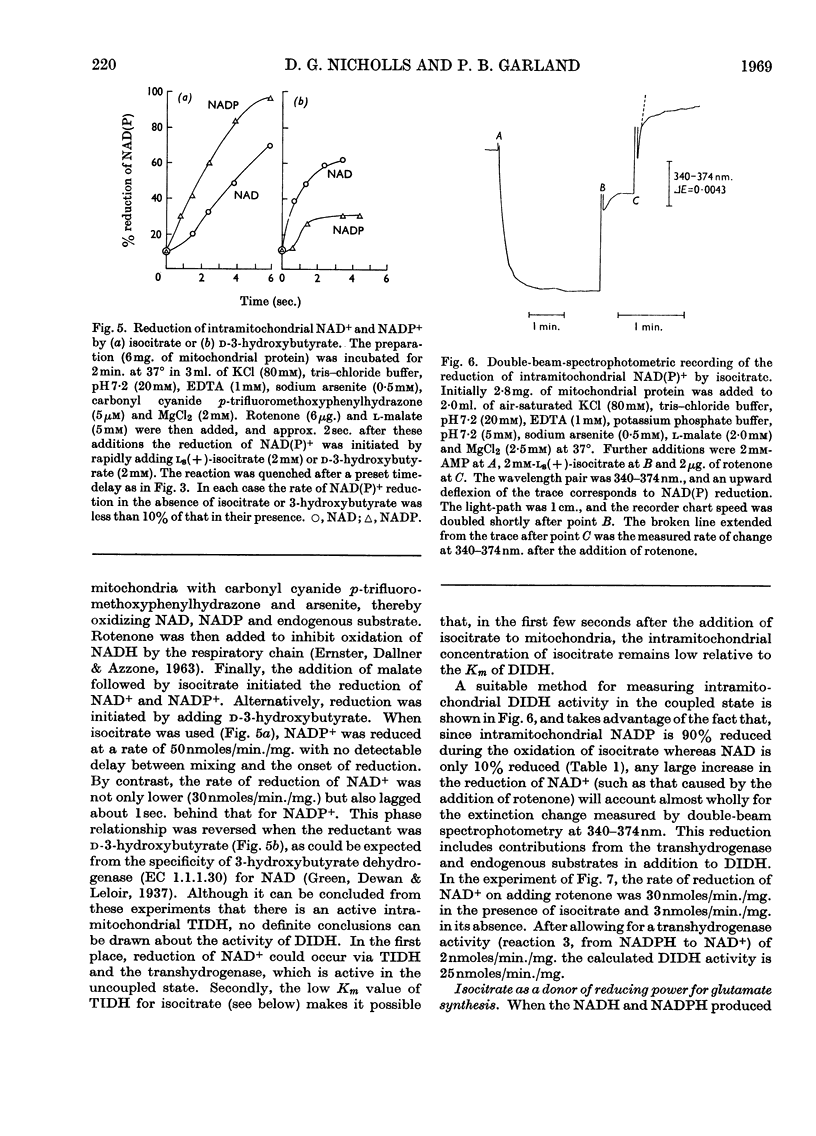


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B., BALTSCHEFFSKY H. Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide. J Biol Chem. 1958 Sep;233(3):736–739. [PubMed] [Google Scholar]
- CHANCE B., HOLLUNGER G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J Biol Chem. 1961 May;236:1534–1543. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- CHEN R. F., PLAUT G. W. ACTIVATION AND INHIBITION OF DPN-LINKED ISOCITRATE DEHYDROGENASE OF HEART BY CERTAIN NUCLEOTIDES. Biochemistry. 1963 Sep-Oct;2:1023–1032. doi: 10.1021/bi00905a020. [DOI] [PubMed] [Google Scholar]
- Chappell J. B., Robinson B. H. Penetration of the mitochondrial membrane by tricarboxylic acid anions. Biochem Soc Symp. 1968;27:123–133. [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERNSTER L. Distribution in interaction of enzymes within animal cells. Biochem Soc Symp. 1959;16:54–72. [PubMed] [Google Scholar]
- ERNSTER L., GLASKY A. J. On the mitochondrial oxidation of isocitrate. Biochim Biophys Acta. 1960 Feb 12;38:168–170. doi: 10.1016/0006-3002(60)91216-6. [DOI] [PubMed] [Google Scholar]
- ERNSTER L., NAVAZIO F. Studies on TPN-linked oxidations. I. Pathways of isocitrate oxidation in rat liver micochondria. Biochim Biophys Acta. 1957 Nov;26(2):408–415. doi: 10.1016/0006-3002(57)90023-9. [DOI] [PubMed] [Google Scholar]
- ERNSTER L., NAVAZIO F. The cytoplasmic distribution of isocitric dehydrogenases. Exp Cell Res. 1956 Aug;11(2):483–486. doi: 10.1016/0014-4827(56)90124-0. [DOI] [PubMed] [Google Scholar]
- GREENSPAN M., PURVIS J. L. ENERGY-LINKED INCORPORATION OF DIPHOSPHOPYRIDINE NUCLEOTIDE INTO RAT-LIVER MITOCHONDRIA. Biochim Biophys Acta. 1965 Apr 26;99:191–194. doi: 10.1016/s0926-6593(65)80026-1. [DOI] [PubMed] [Google Scholar]
- Garland P. B. Control of citrate synthesis in mitochondria. Biochem Soc Symp. 1968;27:41–60. [PubMed] [Google Scholar]
- Garland P. B., Shepherd D., Yates D. W. Steady-state concentrations of coenzyme A, acetyl-coenzyme A and long-chain fatty acyl-coenzyme A in rat-liver mitochondria oxidizing palmitate. Biochem J. 1965 Nov;97(2):587–594. doi: 10.1042/bj0970587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Dewan J. G., Leloir L. F. The beta-hydroxybutyric dehydrogenase of animal tissues. Biochem J. 1937 Jun;31(6):934–949. doi: 10.1042/bj0310934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWTREY A. O. Studies on isocitrate oxidation in mitochondria of normal rat liver and azo-dye-induced hepatomas. Biochem J. 1962 Nov;85:293–305. doi: 10.1042/bj0850293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYTLER P. G. uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry. 1963 Mar-Apr;2:357–361. doi: 10.1021/bi00902a031. [DOI] [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NEUFELD E. F. Pyridine nucleotide transhydrogenase. III. Animal tissue transhydrogenases. J Biol Chem. 1953 Nov;205(1):1–15. [PubMed] [Google Scholar]
- Kaplan N. O., Swartz M. N., Frech M. E., Ciotti M. M. PHOSPHORYLATIVE AND NONPHOSPHORYLATIVE PATHWAYS OF ELECTRON TRANSFER IN RAT LIVER MITOCHONDRIA. Proc Natl Acad Sci U S A. 1956 Aug;42(8):481–487. doi: 10.1073/pnas.42.8.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Shepherd D., Garland P. B. A continuous recording technique for the measurement of carbon dioxide, and its application to mitochondrial oxidation and decarboxylation reactions. Biochem J. 1967 Jun;103(3):677–691. doi: 10.1042/bj1030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PURVIS J. L. Pathway of oxidation of isocitrate by mitochondria. Biochim Biophys Acta. 1958 Nov;30(2):440–441. doi: 10.1016/0006-3002(58)90080-5. [DOI] [PubMed] [Google Scholar]
- Peters R. A., Sinclair H. M., Thompson R. H. An analysis of the inhibition of pyruvate oxidation by arsenicals in relation to the enzyme theory of vesication. Biochem J. 1946;40(4):516–524. [PMC free article] [PubMed] [Google Scholar]
- Plaut G. W., Aogaichi T. The separation of DPN-linked and TPN-linked isocitrate dehydrogenase activities of mammalian liver. Biochem Biophys Res Commun. 1967 Aug 23;28(4):628–634. doi: 10.1016/0006-291x(67)90360-9. [DOI] [PubMed] [Google Scholar]
- STEIN A. M., KAPLAN N. O., CIOTTI M. M. Pyridine nucleotide transhydrogenase. VII. Determination of the reactions with coenzyme analogues in mammalian tissues. J Biol Chem. 1959 Apr;234(4):979–986. [PubMed] [Google Scholar]
- Stein A. M., Kirkman S. K., Stein J. H. Diphosphopyridine nucleotide specific isocitric dehydrogenase of mammalian mitochondria. II. Kinetic properties of the enzyme of the Ehrlich ascites carcinoma. Biochemistry. 1967 Oct;6(10):3197–3203. doi: 10.1021/bi00862a030. [DOI] [PubMed] [Google Scholar]
- Stein A. M., Stein J. H., Kirkman S. K. Diphosphopyridine nucleotide specific isocitric dehydrogenase of mammalian mitochondria. I. On the roles of pyridine nucleotide transhydrogenase and the isocitric dehydrogenases in the respiration of mitochondria of normal and neoplastic tissues. Biochemistry. 1967 May;6(5):1370–1379. doi: 10.1021/bi00857a020. [DOI] [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Membranes and fatty acid metabolism. Br Med Bull. 1968 May;24(2):158–164. doi: 10.1093/oxfordjournals.bmb.a070619. [DOI] [PubMed] [Google Scholar]
- VIGNAIS P. V., VIGNAIS P. M. [Role of di- and triphosphopyridine nucleotides in the mitochondrial oxidation of isocitrate]. Biochim Biophys Acta. 1961 Mar 4;47:515–528. doi: 10.1016/0006-3002(61)90545-5. [DOI] [PubMed] [Google Scholar]
- WEINBACH E. C., SHEFFIELD H., GARBUS J. RESTORATION OF OXIDATIVE PHOSPHORYLATION AND MORPHOLOGICAL INTEGRITY TO SWOLLEN, UNCOUPLED RAT LIVER MITOCHONDRIA. Proc Natl Acad Sci U S A. 1963 Sep;50:561–568. doi: 10.1073/pnas.50.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. W., Garland P. B. The partial latency and intramitochondrial distribution of carnitine-palmitoyltransferase (e.c.2.3.1.-), and the CoASH and carnitine permeable space of rat liver mitochondria. Biochem Biophys Res Commun. 1966 May 25;23(4):460–465. doi: 10.1016/0006-291x(66)90750-9. [DOI] [PubMed] [Google Scholar]


