Abstract
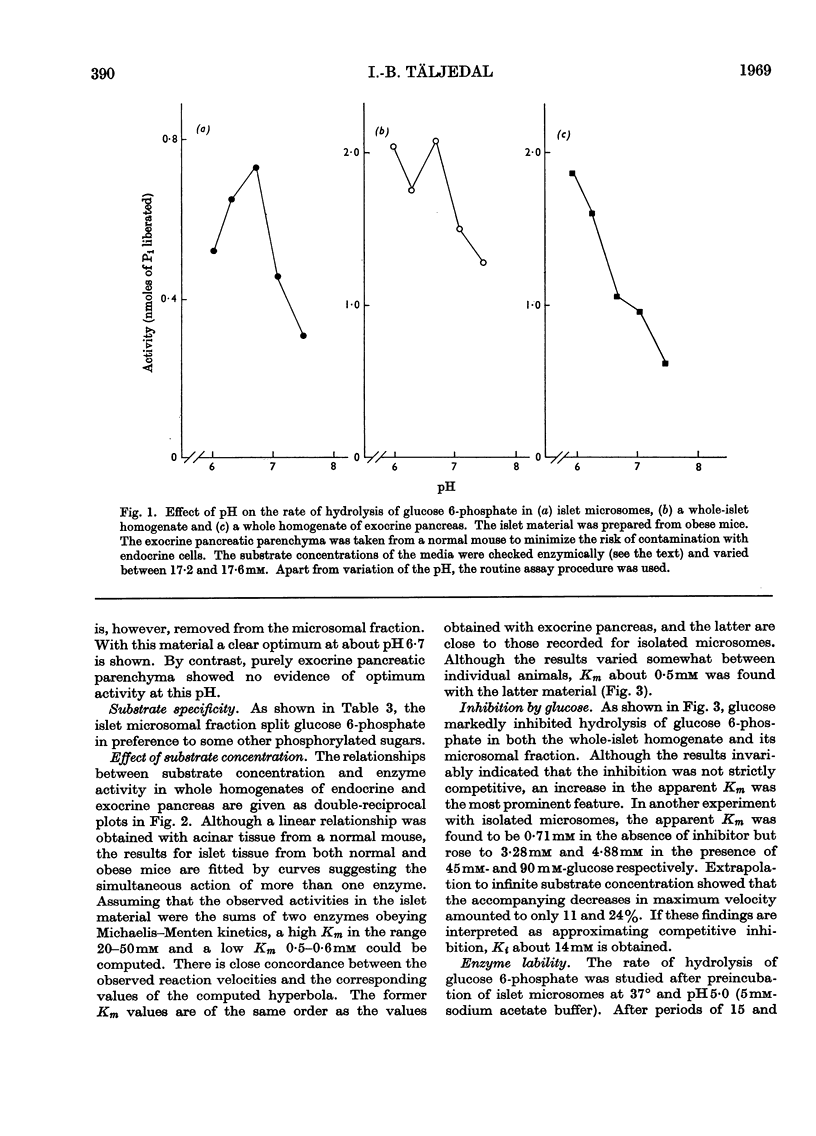
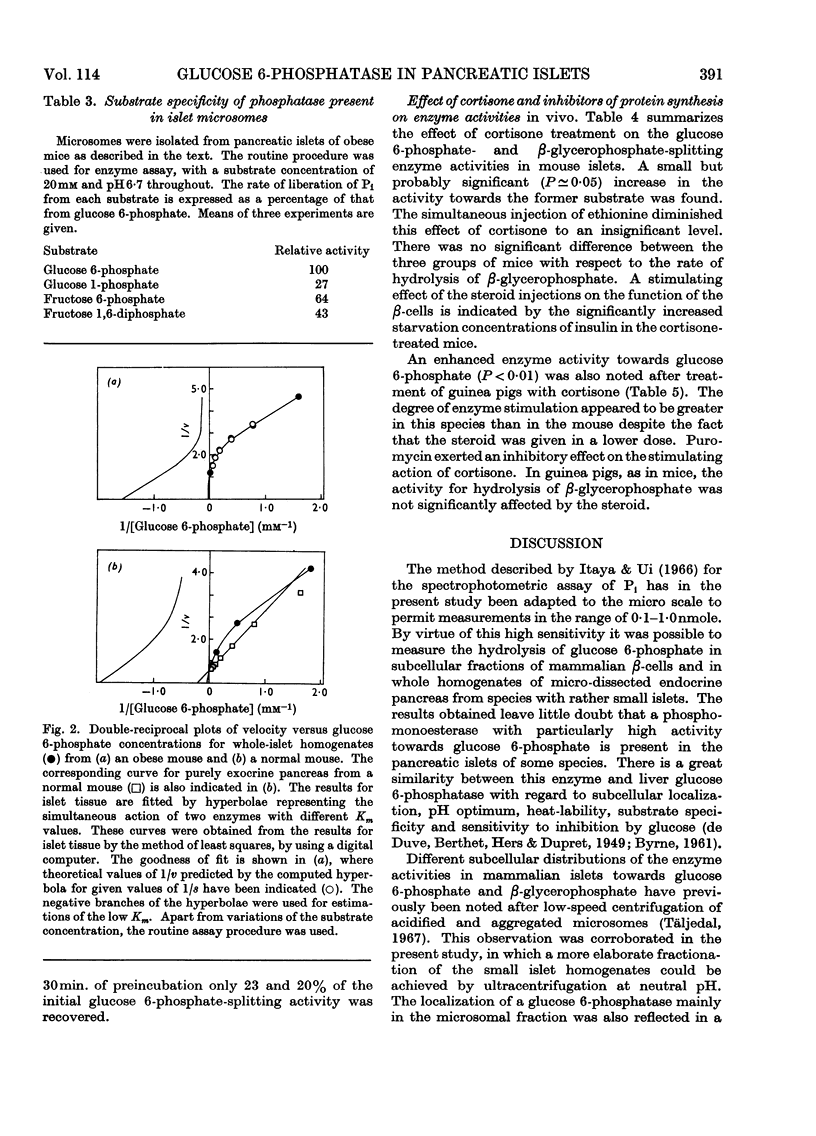
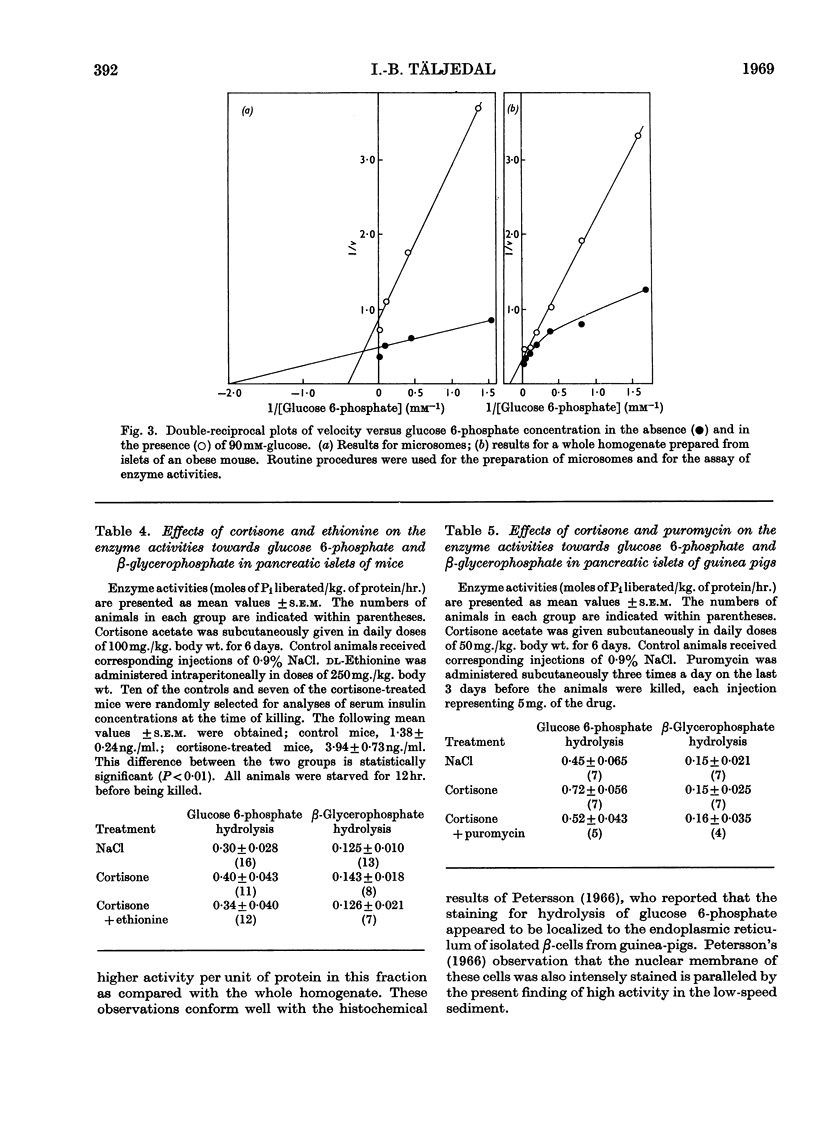
1. Pancreatic islets from several mammalian species were investigated for hydrolytic activity towards glucose 6-phosphate. Both the total phosphatase activity towards this substrate and the proportion cleaving glucose 6-phosphate in preference to β-glycerophosphate varied widely between species. In pancreatic-islet homogenates prepared from mice and guinea pigs there was a higher rate of liberation of Pi at pH6·7 from glucose 6-phosphate than from β-glycerophosphate. In these two species cortisone treatment enhanced the enzyme activity towards glucose 6-phosphate but not that towards β-glycerophosphate. Simultaneous injections of ethionine or puromycin blocked this stimulating effect of cortisone. 2. With whole homogenates of mouse pancreatic islets, inverse plots of the relationship between glucose 6-phosphate concentration and enzyme activity suggested the simultaneous action of two enzymes with different Km values. After fractionation of islets from obese–hyperglycaemic mice by differential centrifugation, one of these enzymes could be shown to be localized in the microsome fraction. It had Km for glucose 6-phosphate about 0·5mm and optimum pH6·7. It split glucose 6-phosphate in preference to β-glycerophosphate, glucose 1-phosphate, fructose 6-phosphate and fructose 1,6-diphosphate. Incubation of the microsomes at pH5·0 and 37° for 15min. decreased the enzyme activity by about 80%. Glucose was a potent inhibitor, the type of inhibition being neither strictly competitive nor non-competitive. It is suggested that the results indicate the presence of glucose 6-phosphatase in mammalian endocrine pancreas, and that this enzyme may play a role in the metabolic regulation of release of insulin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARION W. J., NORDLIE R. C. LIVER MICROSOMAL GLUCOSE 6-PHOSPHATASE, INORGANIC PYROPHOSPHATASE, AND PYROPHOSPHATE-GLUCOSE PHOSPHOTRANSFERASE. II. KINETIC STUDIES. J Biol Chem. 1964 Sep;239:2752–2757. [PubMed] [Google Scholar]
- Ashcroft S. J., Randle P. J. Glucose-6-phosphatase activity of mouse pancreatic islets. Nature. 1968 Aug 24;219(5156):857–858. doi: 10.1038/219857a0. [DOI] [PubMed] [Google Scholar]
- COORE H. G., RANDLE P. J., SIMON E., KRAICER P. F., SHELESNYAK M. C. Block of insulin secretion from the pancreas by D-mannoheptulose. Nature. 1963 Mar 30;197:1264–1266. doi: 10.1038/1971264a0. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLMAN B., HELLERSTROEM C. Histochemical studies on glucose-6-phosphatase, adenosine triphosphatase and amylo phosphorylase in the pancreatic islets of normal and obese-hyperglycaemic mice. Acta Endocrinol (Copenh) 1962 Mar;39:474–482. doi: 10.1530/acta.0.0390474. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A. Presence and mobilization of glycogen in mammalian pancreatic beta cells. Endocrinology. 1969 Jan;84(1):1–8. doi: 10.1210/endo-84-1-1. [DOI] [PubMed] [Google Scholar]
- Hellman B. Studies in obese-hyperglycemic mice. Ann N Y Acad Sci. 1965 Oct 8;131(1):541–558. doi: 10.1111/j.1749-6632.1965.tb34819.x. [DOI] [PubMed] [Google Scholar]
- INGALLS A. M., DICKIE M. M., SNELL G. D. Obese, a new mutation in the house mouse. J Hered. 1950 Dec;41(12):317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- Itaya K., Ui M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin Chim Acta. 1966 Sep;14(3):361–366. doi: 10.1016/0009-8981(66)90114-8. [DOI] [PubMed] [Google Scholar]
- LAZARUS S. S. Demonstration of glucose-6-phosphatase in mammalian pancreas. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:819–822. doi: 10.3181/00379727-101-25108. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malaisse W. J., Lea M. A., Malaisse-Lagae F. The effect of mannoheptulose on the phosphorylation of glucose and the secretion of insulin by islets of Langerhans. Metabolism. 1968 Feb;17(2):126–132. doi: 10.1016/0026-0495(68)90138-8. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968 May 25;243(10):2730–2736. [PubMed] [Google Scholar]
- Petersson B. Cytochemical studies of hydrolytic and oxidative enzymes in sections and isolated cells from the endocrine pancreas of guinea-pigs. Histochemie. 1966;7(2):116–131. doi: 10.1007/BF00309717. [DOI] [PubMed] [Google Scholar]
- Petkov P., Verne J., Wegmann R. Sur l'apparition de glucose-6-phosphatse dans le pancréas du rat blanc au cours du diabète alloxanque. Experientia. 1965 Sep 15;21(9):530–531. doi: 10.1007/BF02138978. [DOI] [PubMed] [Google Scholar]
- SEGAL H. L., WASHKO M. E., LEE C. W. Some kinetic parameters of liver glucose-6-phosphatase in normal and diabetic rats. Science. 1958 Aug 1;128(3318):251–252. doi: 10.1126/science.128.3318.251. [DOI] [PubMed] [Google Scholar]
- Samols E., Marri G., Marks V. Interrelationship of glucagon, insulin and glucose. The insulinogenic effect of glucagon. Diabetes. 1966 Dec;15(12):855–866. doi: 10.2337/diab.15.12.855. [DOI] [PubMed] [Google Scholar]
- Täljedal I. Some aspects of the apparent glucose-6-phosphatase activity in the pancreatic islets of mammals. Biochim Biophys Acta. 1967 Sep 12;146(1):292–295. doi: 10.1016/0005-2744(67)90098-8. [DOI] [PubMed] [Google Scholar]


