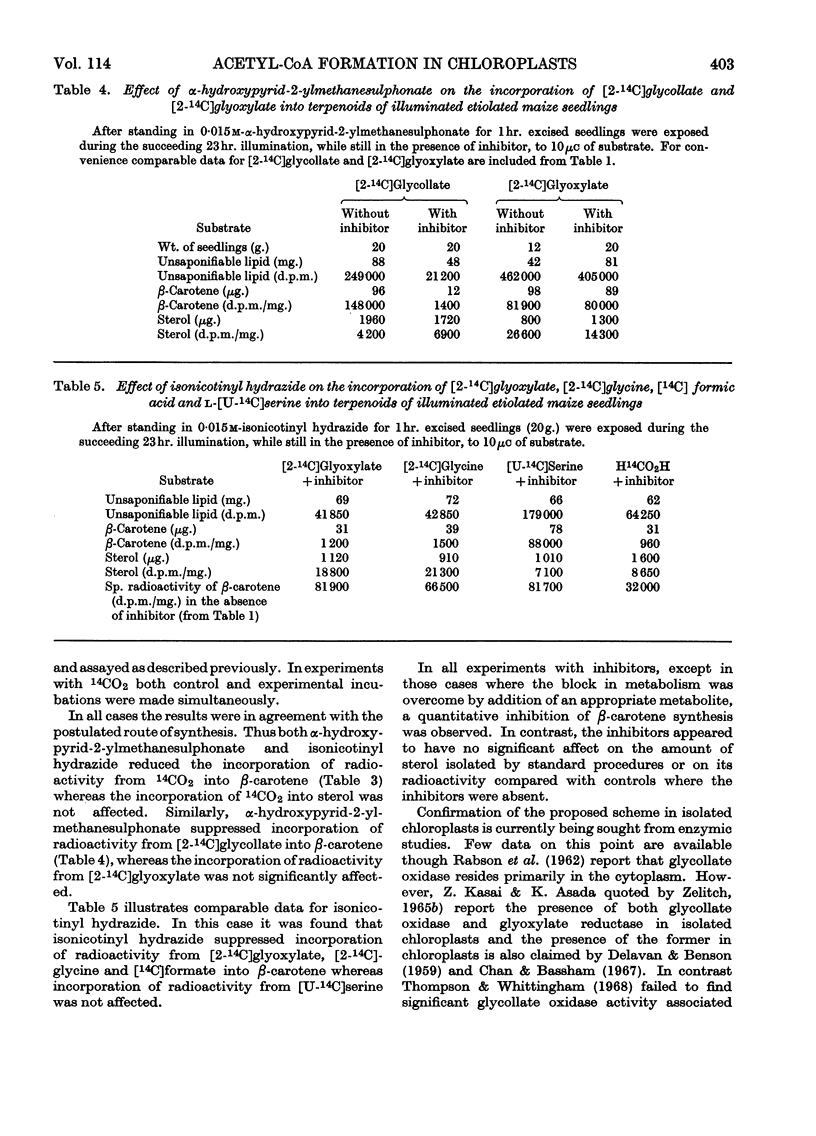
Abstract
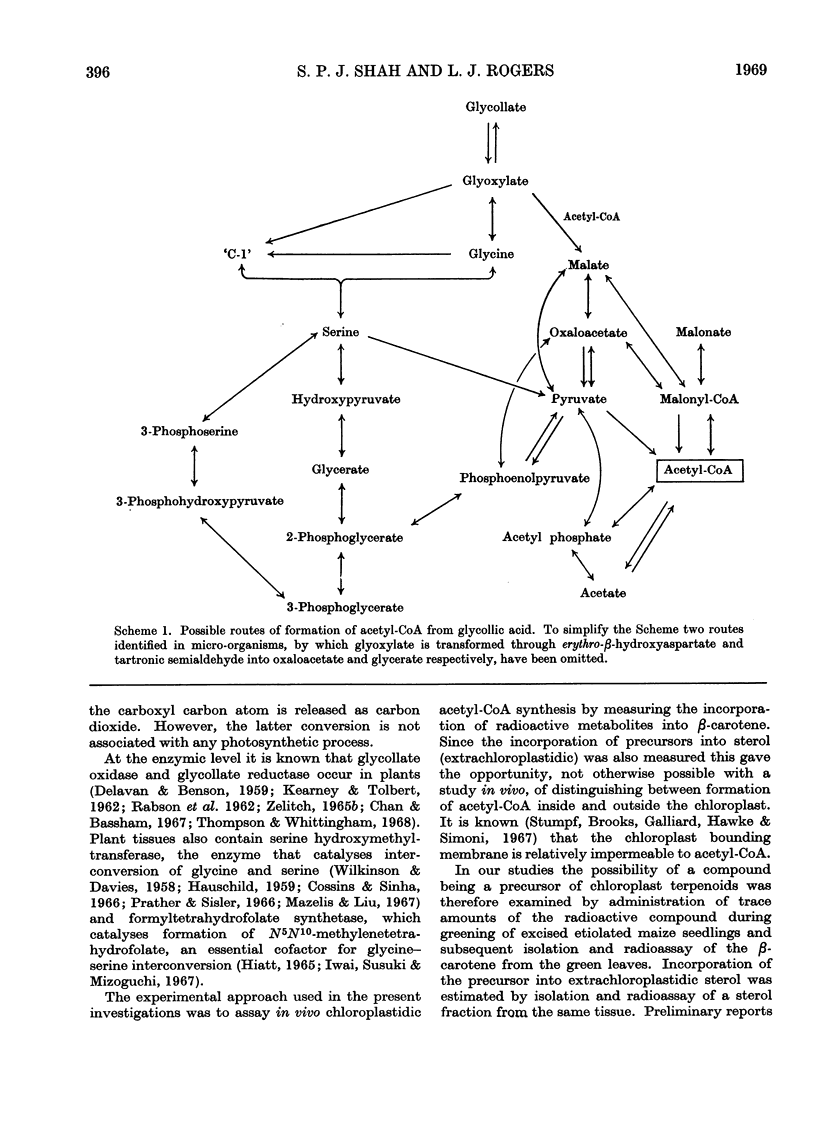
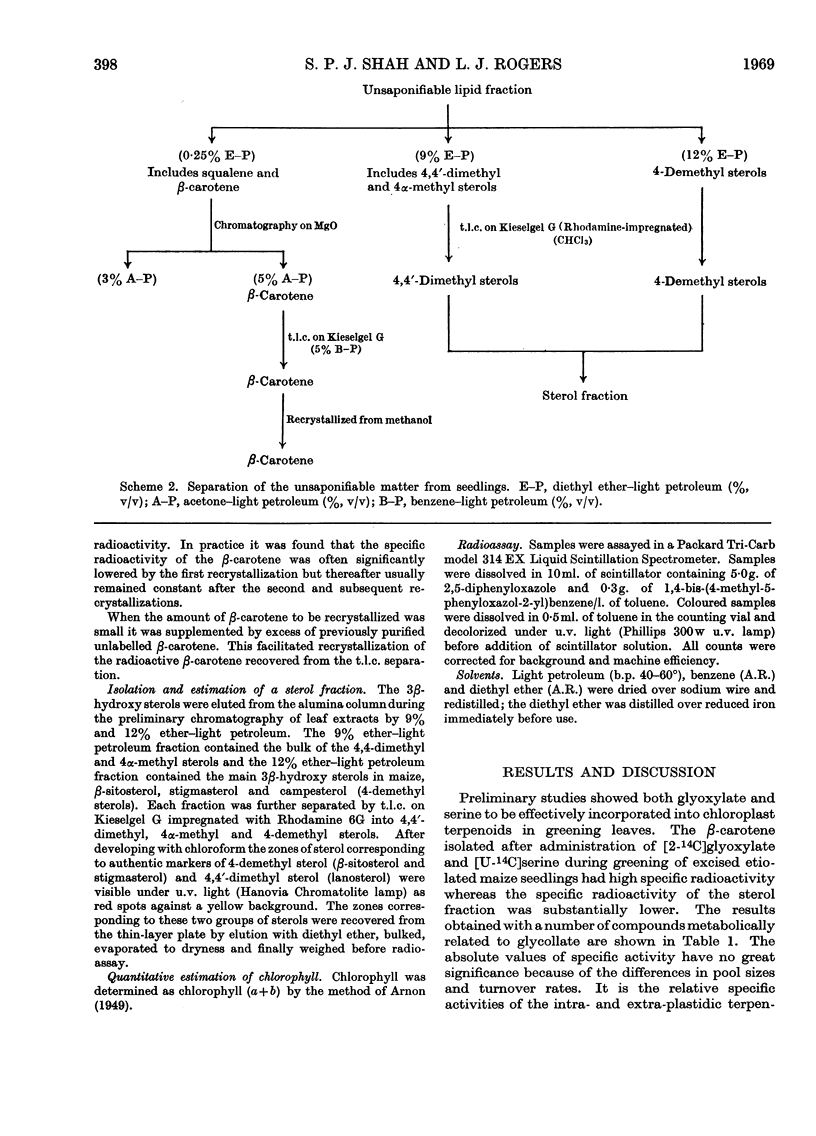
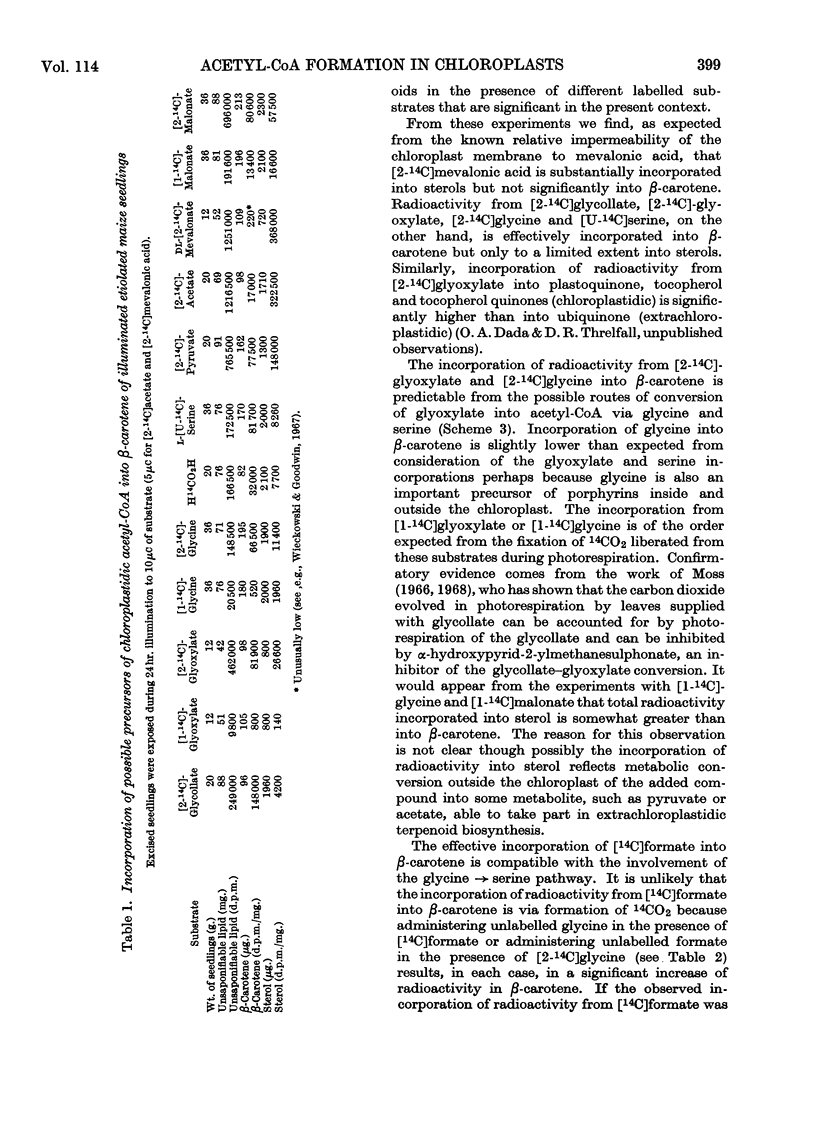
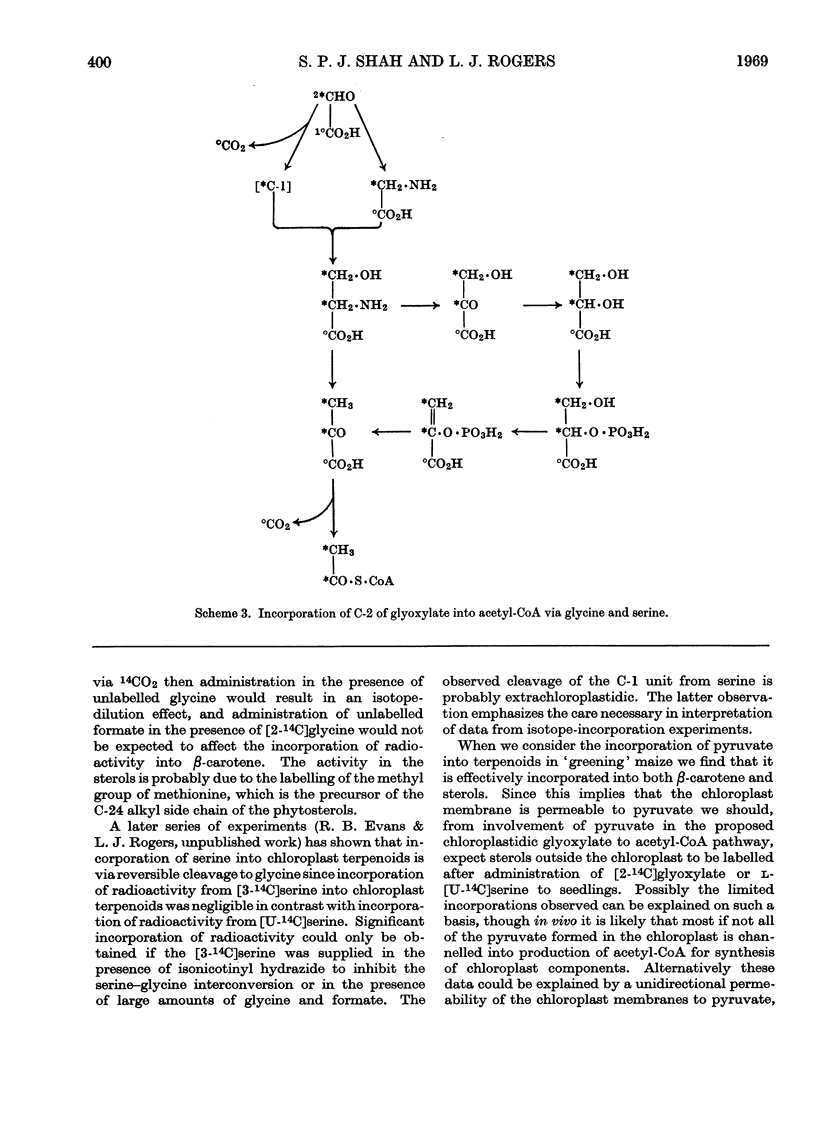
On the basis of radioisotope-incorporation experiments it is suggested that acetyl-CoA, an obligatory intermediate in chloroplast terpenoid biosynthesis, may be formed in maize from photosynthetically fixed carbon dioxide by the route carbon dioxide→glycollate→glyoxylate→glycine→serine→pyruvate→acetyl-CoA. The proposed route is supported by conventional radioisotope-dilution studies and by experiments with inhibitors affecting reactions involved in the pathway. The proposed route appears to play little part in formation of extrachloroplastidic sterol.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRON E. J., SQUIRES C., STUMPF P. K. Fat metabolism in higher plants. XV. Enzymic synthesis of fatty acids by an extract of avocado mesocarp. J Biol Chem. 1961 Oct;236:2610–2614. [PubMed] [Google Scholar]
- BRAITHWAITE G. D., GOODWIN T. W. Studies in carotenogenesis. 25. The incorporation of [1-C14] acetate, [2-C14] acetate and C14-labelled carbon dioxide into lycopene by tomato slices. Biochem J. 1960 Jul;76:1–5. doi: 10.1042/bj0760001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAITHWAITE G. D., GOODWIN T. W. Studies in carotenogenesis. 26. The incorporation of [C14] acetate, [C14] mevalonate and C14-labelled carbon dioxide into beta-carotene by the fungus Phycomyces blakesleeanus. Biochem J. 1960 Jul;76:5–10. doi: 10.1042/bj0760005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAITHWAITE G. D., GOODWIN T. W. Studies in carotenogenesis. 27. Incorporation of [2-14C] acetate, DL-[2-14C] mevalonate and C14-labelled carbon dioxide into carrotroot preparations. Biochem J. 1960 Jul;76:194–197. doi: 10.1042/bj0760194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODIE J. D., WASSON G., PORTER J. W. ENZYME-BOUND INTERMEDIATES IN THE BIOSYNTHESIS OF MEVALONIC AND PALMITIC AICDS. J Biol Chem. 1964 May;239:1346–1356. [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H. W., Bassham J. A. Metabolism of 14C-labeled glycolic acid by isolated spinach chloroplasts. Biochim Biophys Acta. 1967 Jul 25;141(2):426–429. doi: 10.1016/0304-4165(67)90119-5. [DOI] [PubMed] [Google Scholar]
- Cheung G. P., Rosenblum I. Y., Sallach H. J. Comparative studies of enzymes related to serine metabolism in higher plants. Plant Physiol. 1968 Nov;43(11):1813–1820. doi: 10.1104/pp.43.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins E. A., Sinha S. K. The interconversion of glycine and serine by plant tissue extracts. Biochem J. 1966 Nov;101(2):542–549. doi: 10.1042/bj1010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downton W. J., Tregunna E. B. Photorespiration and Glycolate Metabolism: A Re-examination and Correlation of Some Previous Studies. Plant Physiol. 1968 Jun;43(6):923–929. doi: 10.1104/pp.43.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. 25. The incorporation of 14CO2, [2-14C] acetate and [2-14C]mevalonate into beta-carotene by illuminated etiolated maize seedings. Biochem J. 1958 Dec;70(4):612–617. doi: 10.1042/bj0700612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. L., Tolbert N. E. Glycolate, glycine, serine, and glycerate formation during photosynthesis by tobacco leaves. J Biol Chem. 1966 Dec 10;241(23):5705–5711. [PubMed] [Google Scholar]
- Hiatt A. J. Formic Acid Activation in Plants. I. Purification, Properties and Distribution of Formyltetrahydrofolate Synthetase. Plant Physiol. 1965 Jan;40(1):184–188. doi: 10.1104/pp.40.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. J., Kekwick R. G. The role of malonyl-coenzyme A in isoprenoid biosynthesis. Biochem J. 1969 Jul;113(3):36P–36P. doi: 10.1042/bj1130036p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEARNEY P. C., TOLBERT N. E. Appearance of glycolate and related products of photosynthesis outside of chloroplasts. Arch Biochem Biophys. 1962 Jul;98:164–171. doi: 10.1016/0003-9861(62)90162-5. [DOI] [PubMed] [Google Scholar]
- Kay R. E., Phinney B. Plastid Pigment Changes in the Early Seedling Leaves of Zea Mays L. Plant Physiol. 1956 May;31(3):226–231. doi: 10.1104/pp.31.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M. J., Merrett M. J. Glycollate oxidase in Chlorella pyrenoidosa. Biochim Biophys Acta. 1968 Jul 9;159(3):543–544. doi: 10.1016/0005-2744(68)90140-x. [DOI] [PubMed] [Google Scholar]
- MACKINNEY G., CHICHESTER C. O., NAKAYAMA T. The incorporation of glycine carbon into beta-carotene in Phycomyces blakesleeanus. Biochem J. 1955 Aug;60(4):xxxvii–xxxviii. [PubMed] [Google Scholar]
- Mazelis M., Liu E. S. Serine Transhydroxymethylase of Cauliflower (Brassica oleracea var. botrytis L.): Partial Purification and Properties. Plant Physiol. 1967 Dec;42(12):1763–1768. doi: 10.1104/pp.42.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G. M., Tolbert N. E., Jimenez E. Rate of Glycolate Formation During Photosynthesis at High pH. Plant Physiol. 1966 Jan;41(1):143–147. doi: 10.1104/pp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON R., TOLBERTNE, KEARNEY P. C. Formation of serine and glyceric acid by the glycolate pathway. Arch Biochem Biophys. 1962 Jul;98:154–163. doi: 10.1016/0003-9861(62)90161-3. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Perkins H. J. The incorporation of the two carbons of acetate and glycine into the phorbide and phytol moieties of chlorophylls a and b. Biochim Biophys Acta. 1966 Sep 26;127(1):42–46. doi: 10.1016/0304-4165(66)90473-9. [DOI] [PubMed] [Google Scholar]
- SINHA S. K., COSSINS E. A. THE IMPORTANCE OF GLYOXYLATE IN AMINO ACID BIOSYNTHESIS IN PLANTS. Biochem J. 1965 Jul;96:254–261. doi: 10.1042/bj0960254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMIRNOV B. P., RODIONOVA M. A. VOZDE ISTVIE SVETA NA VKLIUCHENIE AMINOKISLOT V BELKI, NUKLEINOVYE KISLOTY I LIPIDY KHLOROPLASTOV V OPYTAKH IN VITRO. Biokhimiia. 1964 May-Jun;29:386–392. [PubMed] [Google Scholar]
- STAFFORD H. A., MAGALDI A., VENNESLAND B. The enzymatic reduction of hydroxypyruvic acid to D-glyceric acid in higher plants. J Biol Chem. 1954 Apr;207(2):621–629. [PubMed] [Google Scholar]
- Shetty A. S., Miller G. W. Influence of Iron Chlorosis on Pigment and Protein Metabolism in Leaves of Nicotiana tabacum L. Plant Physiol. 1966 Mar;41(3):415–421. doi: 10.1104/pp.41.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S. K., Cossins E. A. The metabolism of [14-C]glycine by plant tissues. Biochem J. 1964 Oct;93(1):27–34. doi: 10.1042/bj0930027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANNER H. A., BROWN T. E., EYSTER C., TREHARNE R. W. A manganese dependent photosynthetic process. Biochem Biophys Res Commun. 1960 Aug;3:205–210. doi: 10.1016/0006-291x(60)90224-2. [DOI] [PubMed] [Google Scholar]
- Thompson C. M., Whittingham C. P. Glycollate metabolism in photosynthesising tissue. Biochim Biophys Acta. 1968 Jan 15;153(1):260–269. doi: 10.1016/0005-2728(68)90168-0. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Hess J. L. The effect of hydroxymethanesulfonates on 14-CO-2 photosynthesis by algae. J Biol Chem. 1966 Dec 10;241(23):5712–5715. [PubMed] [Google Scholar]
- Treharne K. J., Mercer E. I., Goodwin T. W. Incorporation of [14C] carbon dioxide and [2-14C] mevalonic acid into terpenoids of higher plants during chloroplast development. Biochem J. 1966 Apr;99(1):239–245. doi: 10.1042/bj0990239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON A. P., DAVIES D. D. Serine-glycine interconversion in plant tissues. Nature. 1958 Apr 12;181(4615):1070–1071. doi: 10.1038/1811070a0. [DOI] [PubMed] [Google Scholar]
- Wang D., Waygood E. R. Carbon metabolism of C-labeled amino acids in wheat leaves. I. A pathway of glyoxylate-serine metabolism. Plant Physiol. 1962 Nov;37(6):826–832. doi: 10.1104/pp.37.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckowski S., Goodwin T. W. Incorporation of DL-[2-14C]mevalonic acid lactone into beta-carotene and the phytol side chain of chlorophyll in cotyledons of four species of pine seedlings. Biochem J. 1967 Oct;105(1):89–92. doi: 10.1042/bj1050089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I. THE RELATION OF GLYCOLIC ACID SYNTHESIS TO THE PRIMARY PHOTOSYNTHETIC CARBOXYLATION REACTION IN LEAVES. J Biol Chem. 1965 May;240:1869–1876. [PubMed] [Google Scholar]
- ZELITCH I. The relationship of glycolic acid to respiration and photosynthesis in tobacco leaves. J Biol Chem. 1959 Dec;234:3077–3081. [PubMed] [Google Scholar]
- Zelitch I., Day P. R. Glycolate oxidase activity in algae. Plant Physiol. 1968 Feb;43(2):289–291. doi: 10.1104/pp.43.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I., Walker D. A. The Role of Glycolic Acid Metabolism in Opening of Leaf Stomata. Plant Physiol. 1964 Sep;39(5):856–862. doi: 10.1104/pp.39.5.856. [DOI] [PMC free article] [PubMed] [Google Scholar]


