Abstract
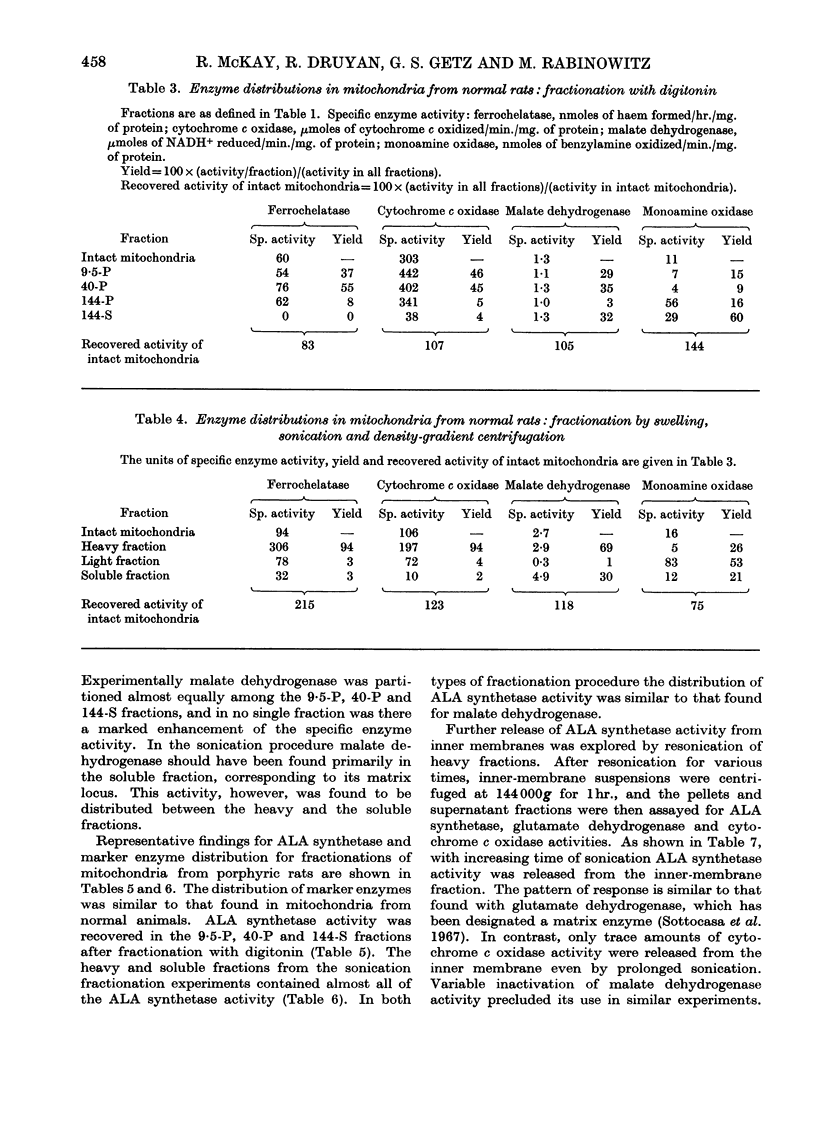
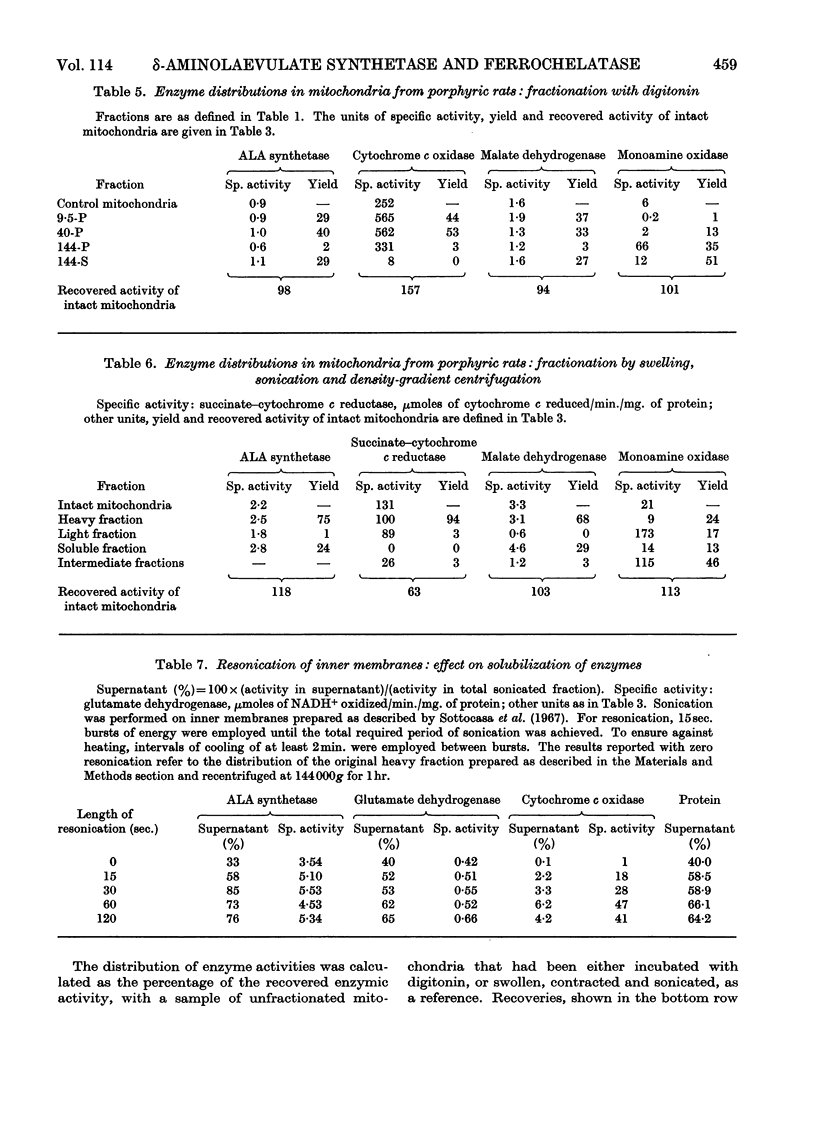
Intramitochondrial loci for δ-aminolaevulate synthetase and ferrochelatase, the initial and final enzymes in haem synthesis, have been found in rat liver. Two different methods of fractionation were applied to mitochondria: (a) sonication and density-gradient centrifugation; (b) treatment with digitonin and differential centrifugation. Similar results were obtained with each technique. δ-Aminolaevulate synthetase is distributed similarly to two known matrix enzymes, malate dehydrogenase and glutamate dehydrogenase. Ferrochelatase is firmly bound to the the inner mitochondrial membrane. These results are considered in terms of the regulation of haem synthesis and in relation to mitochondrial biogenesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie D. S., Basford R. E., Koritz S. B. The turnover of the protein components of mitochondria from rat liver, kidney, and brain. J Biol Chem. 1967 Oct 25;242(20):4584–4586. [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- FIGGE F. H. The in vivo staining of the lining of mouse forestomach by porphyrins and other fluorescent substances. J Histochem Cytochem. 1959 Jul;7(4):257–261. doi: 10.1177/7.4.257. [DOI] [PubMed] [Google Scholar]
- FLETCHER M. J., SANADI D. R. Turnover of rat-liver mitochondria. Biochim Biophys Acta. 1961 Aug 5;51:356–360. doi: 10.1016/0006-3002(61)90177-9. [DOI] [PubMed] [Google Scholar]
- GORNALL A. G., BARDAWILL C. J., DAVID M. M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949 Feb;177(2):751–766. [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Gross N. J., Getz G. S., Rabinowitz M. Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J Biol Chem. 1969 Mar 25;244(6):1552–1562. [PubMed] [Google Scholar]
- Hackenbrock C. R. Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proc Natl Acad Sci U S A. 1968 Oct;61(2):598–605. doi: 10.1073/pnas.61.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. Evidence for the location of ferrochelatase on the inner membrane of rat liver mitochondria. Biochem Biophys Res Commun. 1968 Jun 28;31(6):977–982. doi: 10.1016/0006-291x(68)90549-4. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., HUBBARD N. Preparation and properties of the iron-protoporphyrin chelating enzyme. Biochim Biophys Acta. 1960 Jul 1;41:185–191. doi: 10.1016/0006-3002(60)90001-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marver H. S., Tschudy D. P., Perlroth M. G., Collins A. Delta-aminolevulinic acid synthetase. I. Studies in liver homogenates. J Biol Chem. 1966 Jun 25;241(12):2803–2809. [PubMed] [Google Scholar]
- Munkres K. D., Woodward D. O. On the genetics of enzyme locational specificity. Proc Natl Acad Sci U S A. 1966 May;55(5):1217–1224. doi: 10.1073/pnas.55.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D. F., Williams G. R., Chance B. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann N Y Acad Sci. 1966 Jul 14;137(2):643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ M., DE BERNARD B. Studies on the electron transport system. X. Preparation and spectral properties of a particulate DPNH and succinate cytochrome c reductase from heart muscle. Biochim Biophys Acta. 1957 Oct;26(1):22–29. doi: 10.1016/0006-3002(57)90049-5. [DOI] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- SHEMIN D., KIKUCHI G. Enzymatic synthesis of sigma-aminolevulinic acid. Ann N Y Acad Sci. 1958 Oct 13;75(1):122–128. doi: 10.1111/j.1749-6632.1958.tb36857.x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick R. W., Rexroth A. K., Stange J. L. The metabolism of mitochondrial proteins. 3. The dynamic state of rat liver mitochondria. J Biol Chem. 1968 Jul 10;243(13):3581–3587. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Tschudy D. P., Marver H. S., Collins A. A model for calculating messenger RNA half-life: short lived messenger RNA in the induction of mammalian delta-aminolevulinic acid synthetase. Biochem Biophys Res Commun. 1965 Dec 9;21(5):480–487. doi: 10.1016/0006-291x(65)90408-0. [DOI] [PubMed] [Google Scholar]


