Abstract
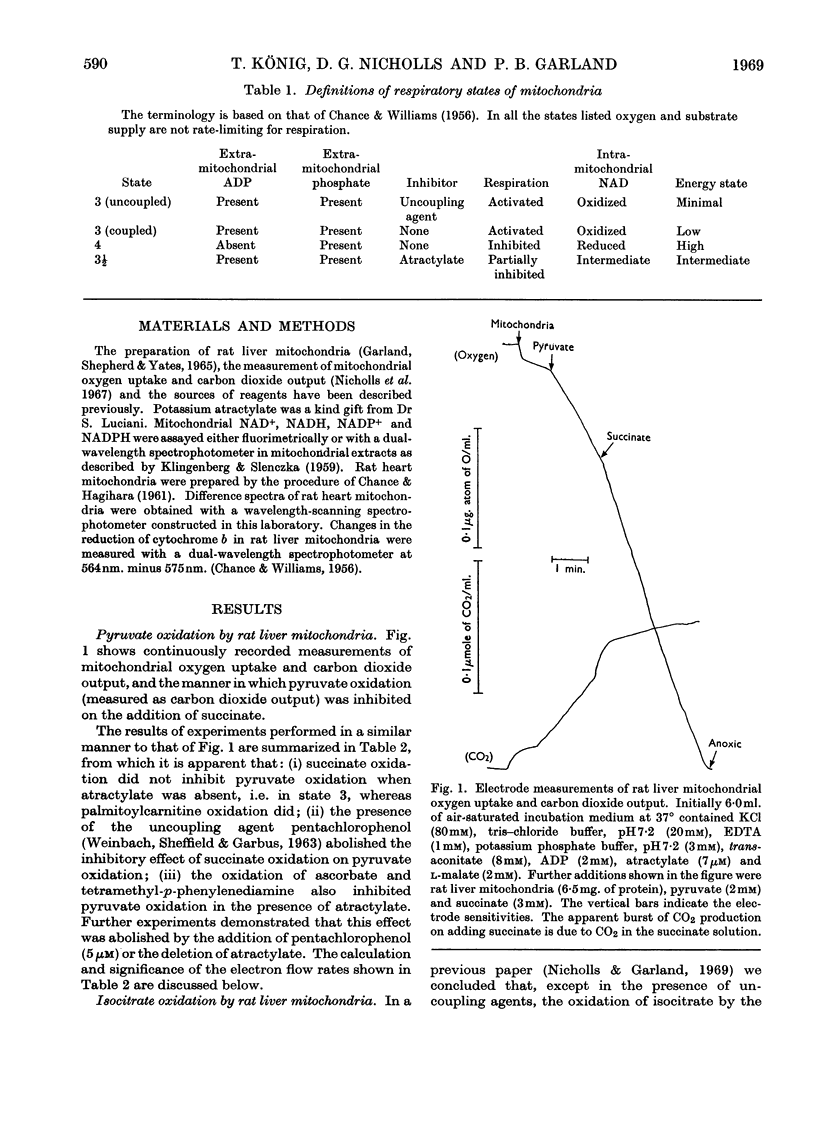
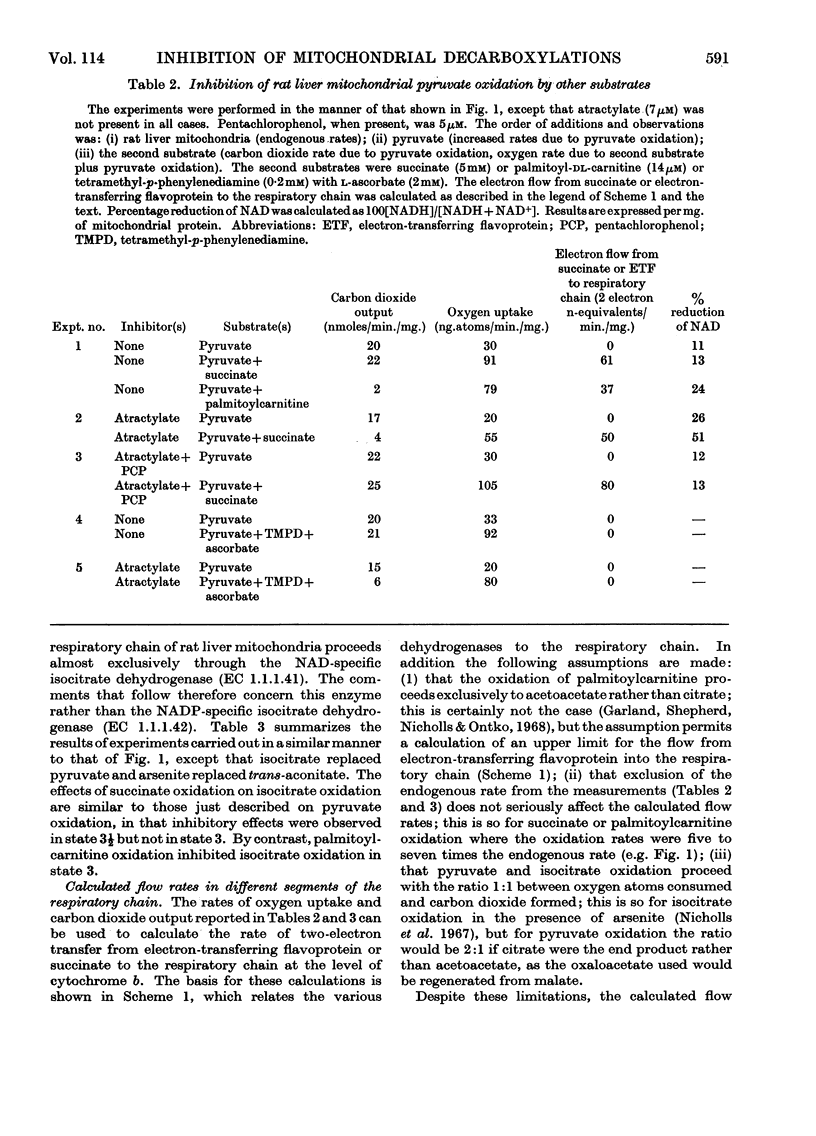
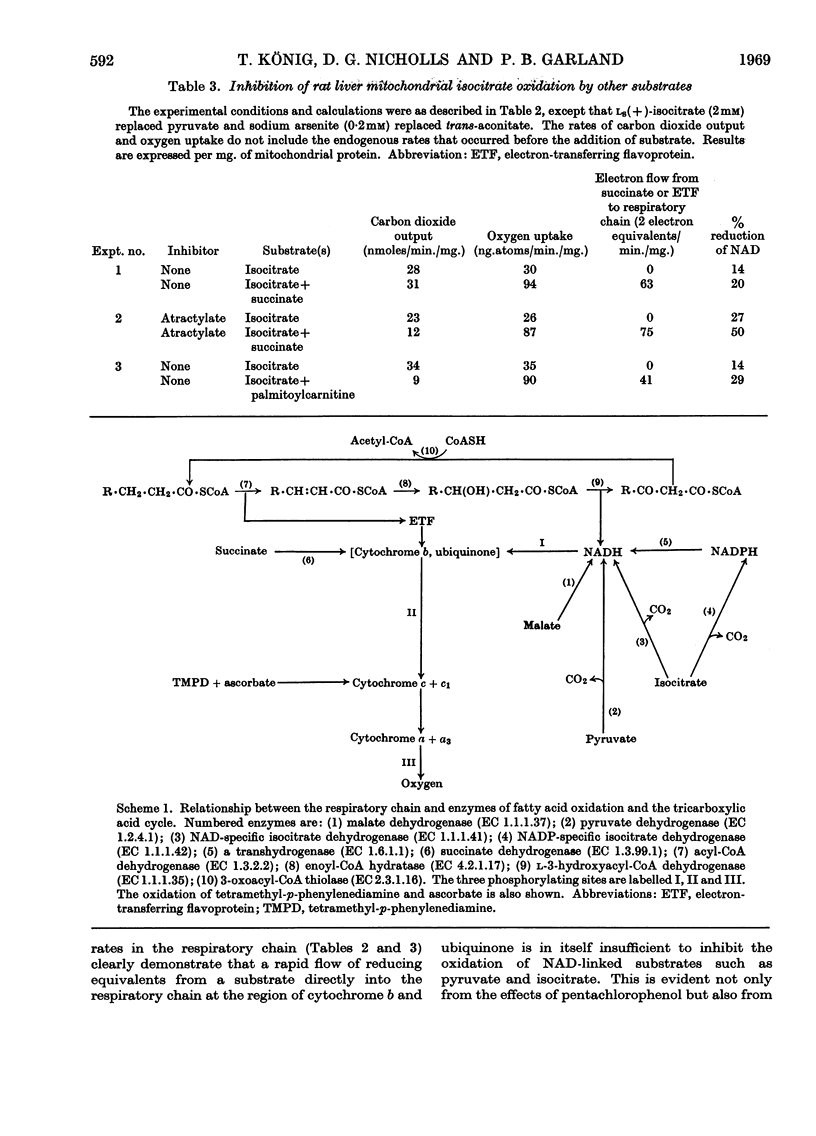
1. The effects of succinate oxidation on pyruvate and also isocitrate oxidation by rat liver mitochondria were studied. 2. Succinate oxidation was without effect on pyruvate and isocitrate oxidation when respiration was maximally activated with ADP. 3. When respiration was partially inhibited by atractylate, succinate oxidation severely inhibited the oxidation of pyruvate and isocitrate. 4. This inhibitory effect of succinate was associated with a two- to three-fold increase in the reduction of mitochondrial NAD+ but no change in the reduction of cytochrome b. 5. It is concluded that, in the partially energy-controlled state, respiration is more severely inhibited at the first phosphorylating site than at the other two. 6. The effects of succinate oxidation are compared with those of palmitoylcarnitine oxidation. It is concluded that a rapid flow of electrons directly into the respiratory chain at the level of cytochrome b is in itself inadequate to inhibit the oxidation of intramitochondrial NADH. 7. The effects of succinate oxidation on pyruvate oxidation were similar in rat heart and liver mitochondria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremer J. Comparison of acylcarnitines and pyruvate as substrates for rat-liver mitochondria. Biochim Biophys Acta. 1966 Feb 1;116(1):1–11. doi: 10.1016/0005-2760(66)90087-7. [DOI] [PubMed] [Google Scholar]
- Bremer J. Pyruvate dehydrogenase, substrate specificity and product inhibition. Eur J Biochem. 1969 Apr;8(4):535–540. doi: 10.1111/j.1432-1033.1969.tb00559.x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. II. Difference spectra. J Biol Chem. 1955 Nov;217(1):395–407. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- GARLAND P. B., NEWSHOLME E. A., RANDLE P. J. Effect of fatty acids, ketone bodies, diabetes and starvation on pyruvate metabolism in rat heart and diaphragm muscle. Nature. 1962 Jul 28;195:381–383. doi: 10.1038/195381a0. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Shepherd D., Nicholls D. G., Ontko J. Energy-dependent control of the tricarboxylic acid cycle by fatty acid oxidation in rat liver mitochondria. Adv Enzyme Regul. 1968;6:3–30. doi: 10.1016/0065-2571(68)90005-8. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Shepherd D., Yates D. W. Steady-state concentrations of coenzyme A, acetyl-coenzyme A and long-chain fatty acyl-coenzyme A in rat-liver mitochondria oxidizing palmitate. Biochem J. 1965 Nov;97(2):587–594. doi: 10.1042/bj0970587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLAM R. J., KREBS H. A. Substrate competition in the respiration of animal tissues. The metabolic interactions of pyruvate and alpha-oxoglutarate in rat-liver homogenates. Biochem J. 1963 Mar;86:432–446. doi: 10.1042/bj0860432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINGENBERG M., SLENCZKA W. [Pyridine nucleotide in liver mitochondria. An analysis of their redox relationships]. Biochem Z. 1959;331:486–517. [PubMed] [Google Scholar]
- KOENIG T., MAROSVARI I., LIPCSEY A. PYRUVATE METABOLISM IN LIVER MITOCHONDRIA. Acta Physiol Acad Sci Hung. 1964;24:391–402. [PubMed] [Google Scholar]
- Klingenberg M., Pfaff E. Metabolic control in mitochondria by adenine nucleotide translocation. Biochem Soc Symp. 1968;27:105–122. [PubMed] [Google Scholar]
- Light P. A., Ragan C. I., Clegg R. A., Garland P. B. Iron-limited growth of torulopsis utilis, and the reversible loss of mitochondrial energy conservation at site 1 and of sensitivity to rotenone and piericidin A. FEBS Lett. 1968 Jul;1(1):4–8. doi: 10.1016/0014-5793(68)80004-3. [DOI] [PubMed] [Google Scholar]
- Nicholls DG RAND P. B. Th control of isocitrate oxidation by rat liver mitochondria. Biochem J. 1969 Sep;114(2):215–225. doi: 10.1042/bj1140215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut G. W., Aogaichi T. The separation of DPN-linked and TPN-linked isocitrate dehydrogenase activities of mammalian liver. Biochem Biophys Res Commun. 1967 Aug 23;28(4):628–634. doi: 10.1016/0006-291x(67)90360-9. [DOI] [PubMed] [Google Scholar]
- SLATER E. C. Mechanism of phosphorylation in the respiratory chain. Nature. 1953 Nov 28;172(4387):975–978. doi: 10.1038/172975a0. [DOI] [PubMed] [Google Scholar]
- WEINBACH E. C., SHEFFIELD H., GARBUS J. RESTORATION OF OXIDATIVE PHOSPHORYLATION AND MORPHOLOGICAL INTEGRITY TO SWOLLEN, UNCOUPLED RAT LIVER MITOCHONDRIA. Proc Natl Acad Sci U S A. 1963 Sep;50:561–568. doi: 10.1073/pnas.50.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Paetkau V., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. 3. The role and regulation of mitochondrial processes involved in supplying precursors of phosphoenolpyruvate. J Biol Chem. 1966 Jun 10;241(11):2523–2532. [PubMed] [Google Scholar]


