Abstract
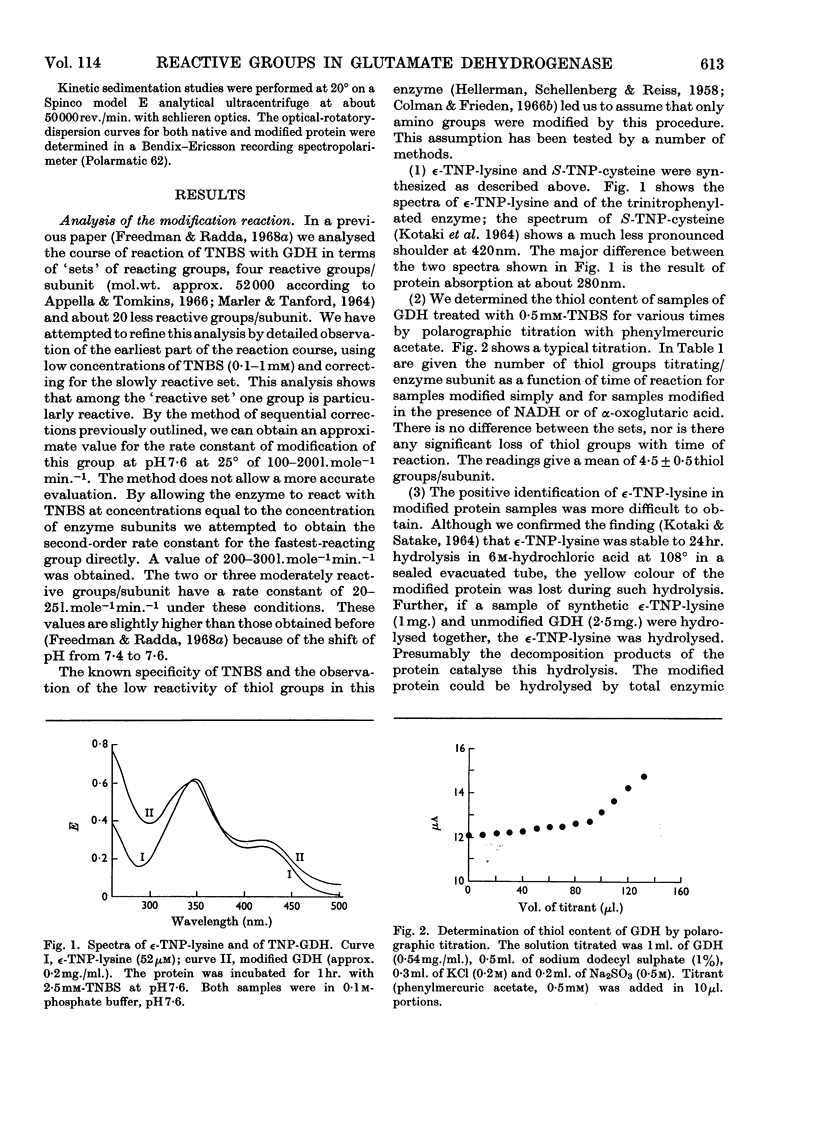
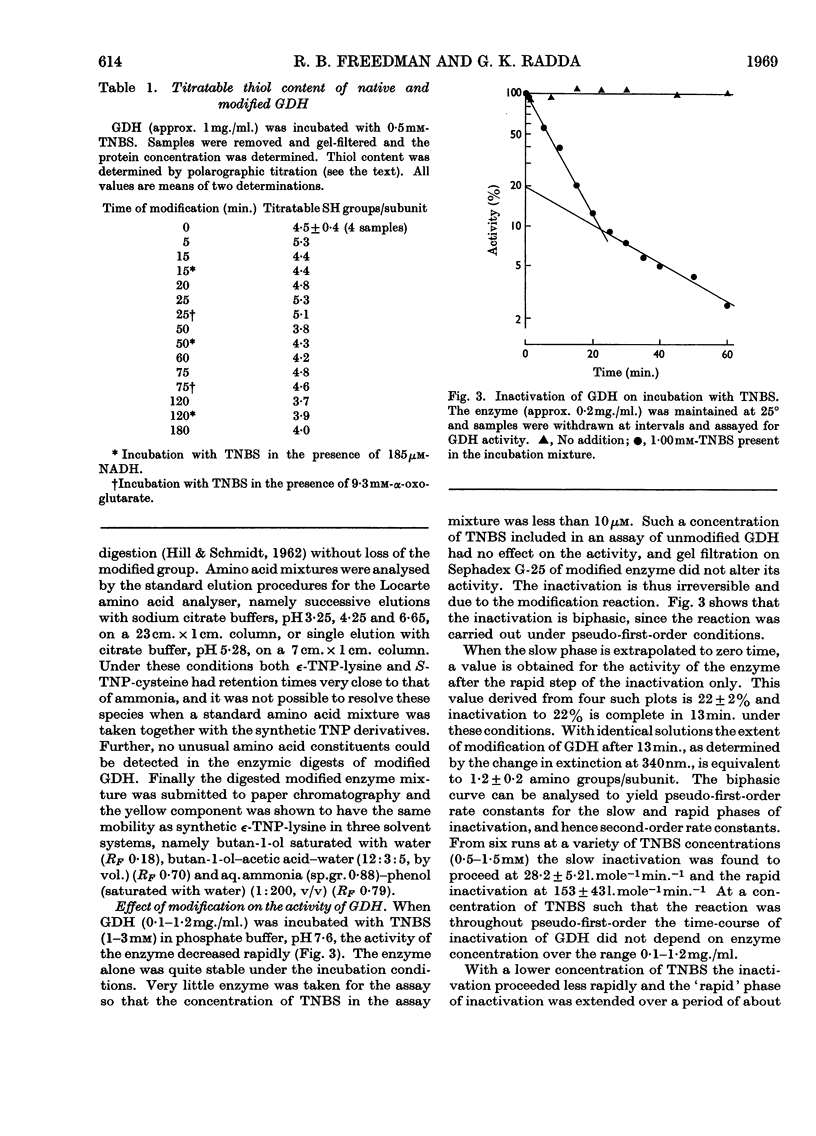
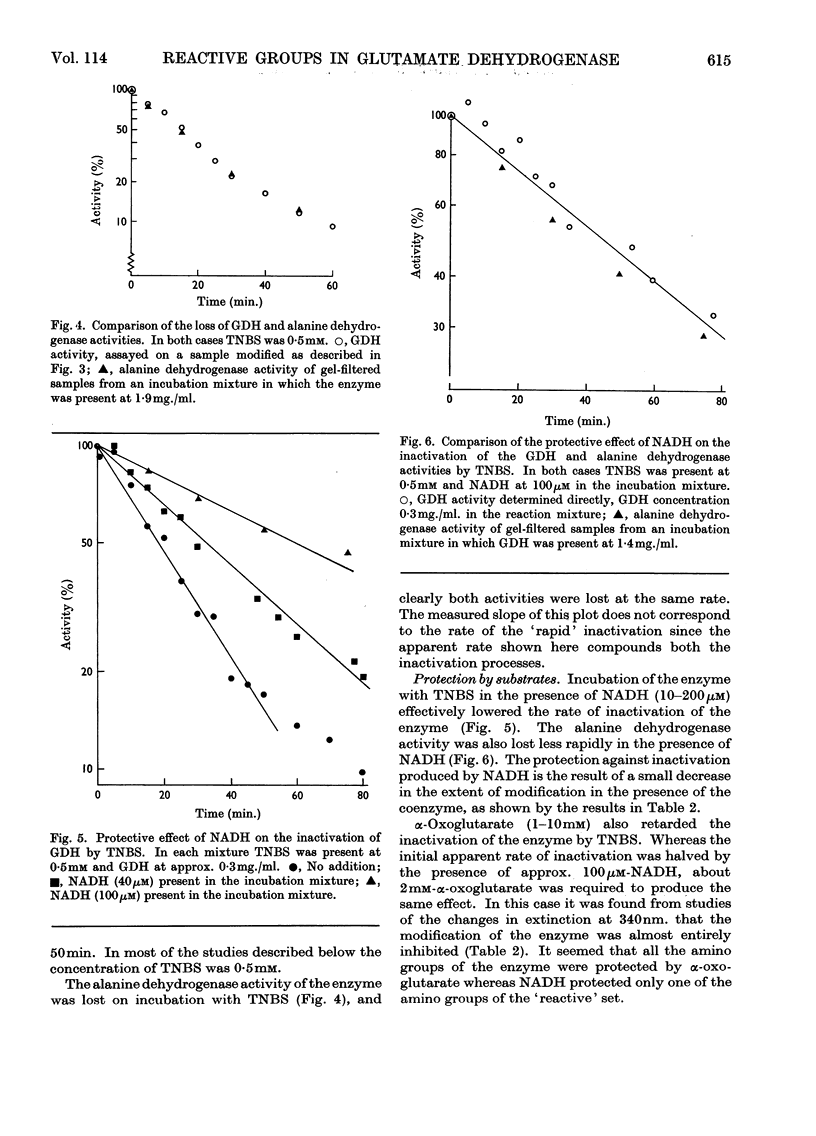
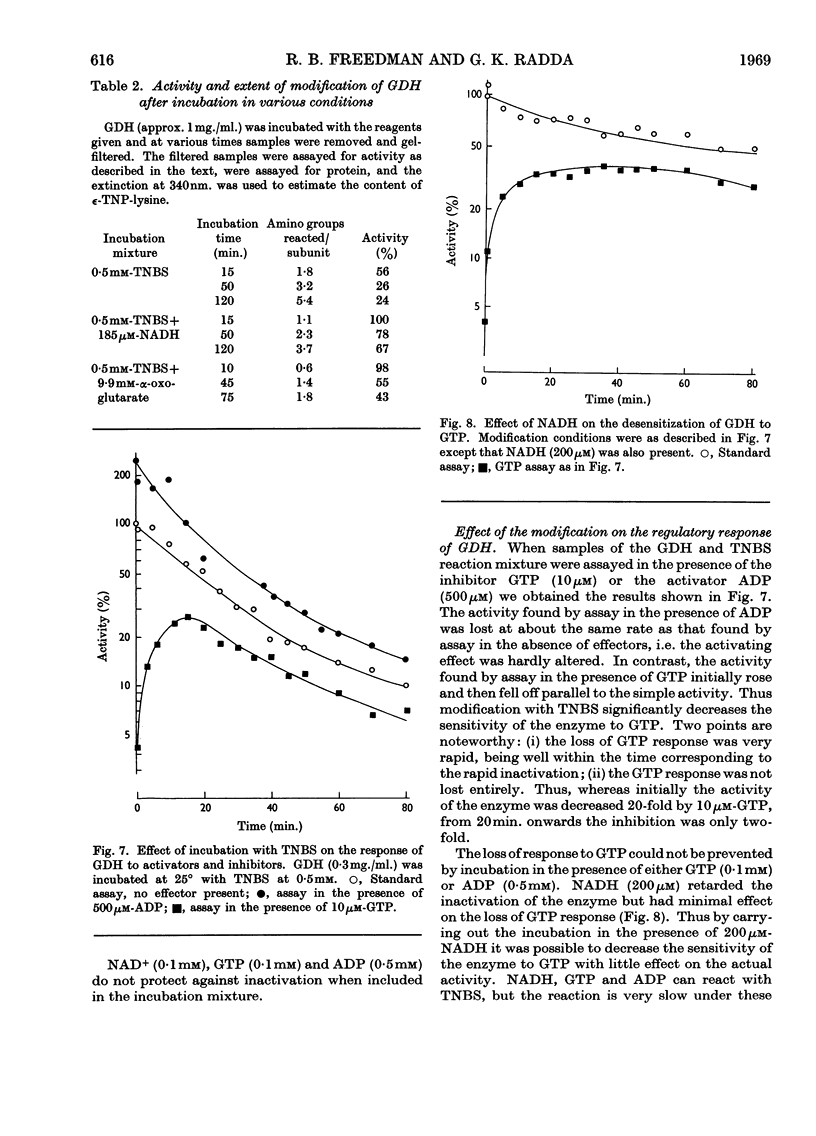
1. Modification with 2,4,6-trinitrobenzenesulphonic acid was studied for its effect on the structure, activity and response to regulatory effectors of ox liver glutamate dehydrogenase. 2. The modification affected amino groups only, and the relative reactivities of the amino groups of the enzyme are described. 3. A biphasic inactivation of the enzyme was observed and analysis of the course of inactivation and of modification showed that the rapid reaction of one amino group/subunit leads to loss of 80% of the enzymic activity. 4. NADH retarded the inactivation by 2,4,6-trinitrobenzenesulphonic acid, the protection increasing with NADH concentration. This, together with the previous observation, suggests that the rapidly reacting group is essential for the activity of the enzyme. 5. The effects of modification on the optical-rotatory-dispersion and sedimentation behaviour of the enzyme were studied. 6. The enzyme's response to the allosteric effector GTP was rapidly lost on modification, whereas its response to ADP was unaffected. Comparison of the inactivation and desensitization suggests that the reactive amino group is essential for both activity and GTP response, and that only a completely unmodified enzyme oligomer responds fully to GTP. 7. The merits of chemical-modification studies of large enzymes are discussed critically in connexion with the interpretation of these results.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. M., Anderson C. D., Churchich J. E. Inhibition of glutamic dehydrogenase by pyridoxal 5'-phosphate. Biochemistry. 1966 Sep;5(9):2893–2900. doi: 10.1021/bi00873a017. [DOI] [PubMed] [Google Scholar]
- Bayley P. M., Radda G. K. Conformational changes and the regulation of glutamate-dehydrogenase activity. Biochem J. 1966 Jan;98(1):105–111. doi: 10.1042/bj0980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CECIL R., SNOW N. S. The reaction of normal adult human haemoglobin with heavy-metal reagents. Biochem J. 1962 Feb;82:247–255. doi: 10.1042/bj0820247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSS D. G., FISHER H. F. SPECTROPHOTOMETRIC STUDIES OF THE QUATERNARY STRUCTURE OF PROTEINS. II. THE ALPHA, BETA SPLIT OF GLUTAMIC DEHYDROGENASE. Arch Biochem Biophys. 1965 Apr;110:222–226. doi: 10.1016/0003-9861(65)90178-5. [DOI] [PubMed] [Google Scholar]
- Cohen L. A. Group-specific reagents in protein chemistry. Annu Rev Biochem. 1968;37:695–726. doi: 10.1146/annurev.bi.37.070168.003403. [DOI] [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. I. Effect of acetylation on catalytic and regulatory properties. J Biol Chem. 1966 Aug 25;241(16):3652–3660. [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. II. Effect of acetylation on molecular properties. J Biol Chem. 1966 Aug 25;241(16):3661–3670. [PubMed] [Google Scholar]
- Dalziel Keith, Engel Paul C. Antagonistic homotropic interactions as a possible explanation of coenzyme activation of glutamate dehydrogenase. FEBS Lett. 1968 Oct;1(5):349–352. doi: 10.1016/0014-5793(68)80153-x. [DOI] [PubMed] [Google Scholar]
- Dodd G. H., Radda G. K. Interaction of glutamate dehydrogenase with fluorescent dyes. Biochem Biophys Res Commun. 1967 May 25;27(4):500–504. doi: 10.1016/s0006-291x(67)80014-7. [DOI] [PubMed] [Google Scholar]
- Doddgh, Radda G. K. 1-Anilinonaphthalene-8-sulphonate, a fluorescent conformational probe for glutamate dehydrogenase. Biochem J. 1969 Sep;114(2):407–417. doi: 10.1042/bj1140407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger M. J., Hirs C. H. On the structure of 41-Dinitrophenyl ribonuclease A. Solvent perturbation, thermal transition, optical rotatory dispersion, and binding studies. Biochemistry. 1968 Oct;7(10):3374–3380. doi: 10.1021/bi00850a010. [DOI] [PubMed] [Google Scholar]
- FISHER H. F., CROSS D. G., McGREGOR L. L. Catalytic activity of sub-units of glutamic dehydrogenase. Nature. 1962 Dec 1;196:895–896. doi: 10.1038/196895b0. [DOI] [PubMed] [Google Scholar]
- FRIEDEN C. GLUTAMATE DEHYDROGENASE. V. THE RELATION OF ENZYME STRUCTURE TO THE CATALYTIC FUNCTION. J Biol Chem. 1963 Oct;238:3286–3299. [PubMed] [Google Scholar]
- Freedman R. B., Radda G. K. The reaction of 2,4,6-trinitrobenzenesulphonic acid with amino acids, Peptides and proteins. Biochem J. 1968 Jul;108(3):383–391. doi: 10.1042/bj1080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden C., Colman R. F. Glutamate dehydrogenase concentration as a determinant in the effect of purine nucleotides on enzymatic activity. J Biol Chem. 1967 Apr 25;242(8):1705–1715. [PubMed] [Google Scholar]
- Goldfarb A. R. A kinetic study of the reactions of amino acids and peptides with trinitrobenzenesulfonic acid. Biochemistry. 1966 Aug;5(8):2570–2574. doi: 10.1021/bi00872a013. [DOI] [PubMed] [Google Scholar]
- HELLERMAN L., SCHELLENBERG K. A., REISS O. K. L-glutamic acid dehydrogenase. II. Role of enzyme sulfhydryl groups. J Biol Chem. 1958 Dec;233(6):1468–1478. [PubMed] [Google Scholar]
- HILL R. L., SCHMIDT W. R. The complete enzymic hydrolysis of proteins. J Biol Chem. 1962 Feb;237:389–396. [PubMed] [Google Scholar]
- HIRS C. H. Dinitrophenyiribonucleases. Brookhaven Symp Biol. 1962 Dec;15:154–183. [PubMed] [Google Scholar]
- KOTAKI A., HARADA M., YAGI K. REACTION BETWEEN SULFHYDRYL COMPOUNDS AND 2,4,6-TRINITROBENZENE-1-SULFONIC ACID. J Biochem. 1964 May;55:553–561. [PubMed] [Google Scholar]
- KOTAKI A., SATAKE K. ACID AND ALKALINE DEGRADATION OF THE TNP-AMINO ACIDS AND -PEPTIDES. J Biochem. 1964 Oct;56:299–307. doi: 10.1093/oxfordjournals.jbchem.a127993. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARLER E., TANFORD C. THE MOLECULAR WEIGHT OF THE POLYPEPTIDE CHAINS OF L-GLUTAMATE DEHYDROGENASE. J Biol Chem. 1964 Dec;239:4217–4218. [PubMed] [Google Scholar]
- Malcolm A. D., Radda G. K. Allosteric transitions of glutamate dehydrogenase. Nature. 1968 Aug 31;219(5157):947–949. doi: 10.1038/219947a0. [DOI] [PubMed] [Google Scholar]
- OLSON J. A., ANFINSEN C. B. The crystallization and characterization of L-glutamic acid dehydrogenase. J Biol Chem. 1952 May;197(1):67–79. [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr A method for characterizing the type and numbers of groups involved in enzyme action. J Biol Chem. 1961 Jul;236:1973–1979. [PubMed] [Google Scholar]
- SRI RAM J., BIER M., MAURER P. H. Chemical modifications of proteins and their significance in enzymology, immunochemistry, and related subjects. Adv Enzymol Relat Subj Biochem. 1962;24:105–160. doi: 10.1002/9780470124888.ch3. [DOI] [PubMed] [Google Scholar]
- Sund H., Burchard W. Sedimentation coefficient and molecular weight of beef liver glutamate dehydrogenase at the microgram and the milligram level. Eur J Biochem. 1968 Nov;6(2):202–206. doi: 10.1111/j.1432-1033.1968.tb00438.x. [DOI] [PubMed] [Google Scholar]
- TOMKINS G. M., YIELDING K. L. Regulation of the enzymic activity of glutamic dehydrogenase mediated by changes in its structure. Cold Spring Harb Symp Quant Biol. 1961;26:331–341. doi: 10.1101/sqb.1961.026.01.040. [DOI] [PubMed] [Google Scholar]
- TSOU C. L. Relation between modification of functional groups of proteins and their biological activity. I.A graphical method for the determination of the number and type of essential groups. Sci Sin. 1962 Nov;11:1535–1558. [PubMed] [Google Scholar]


