Abstract
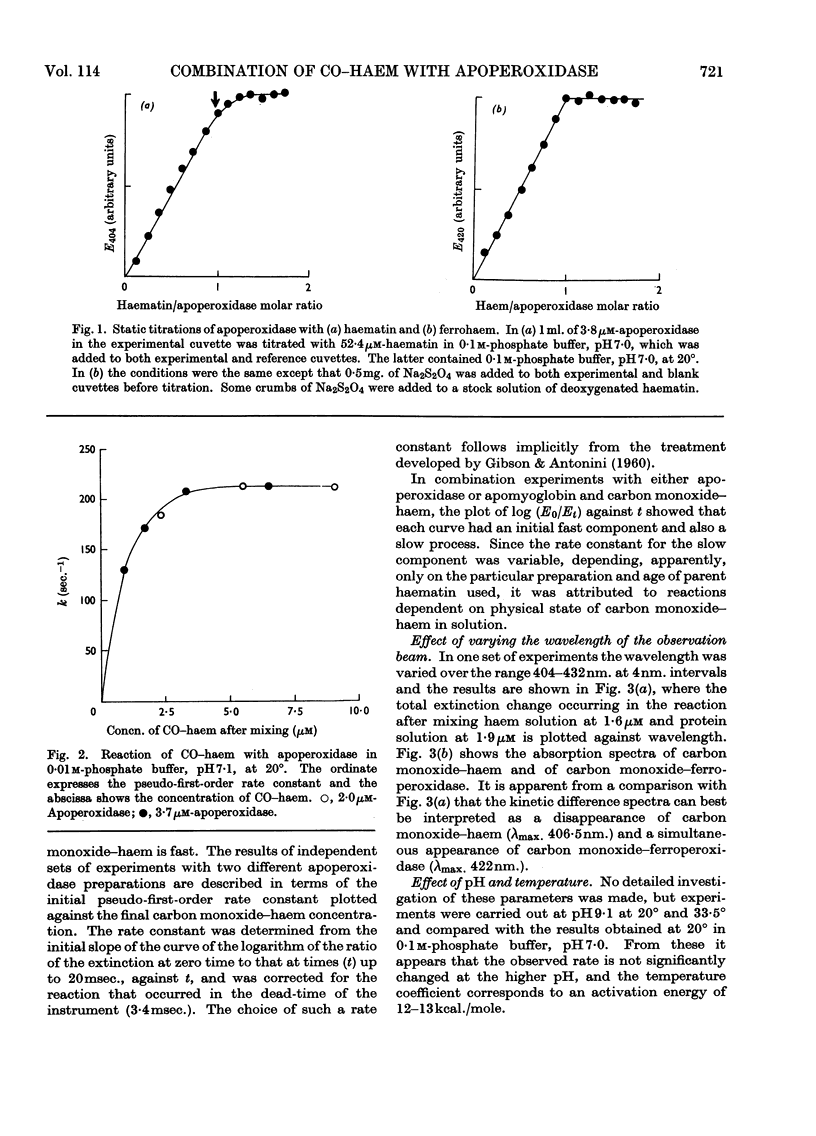
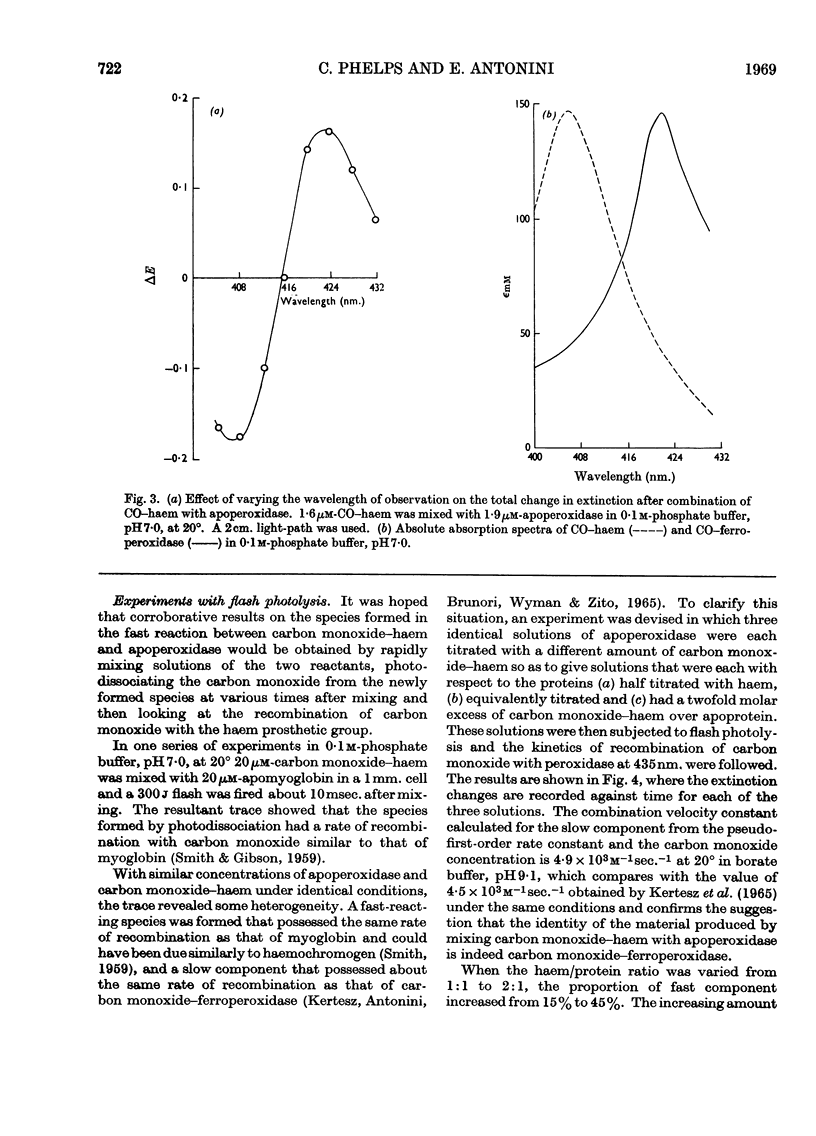
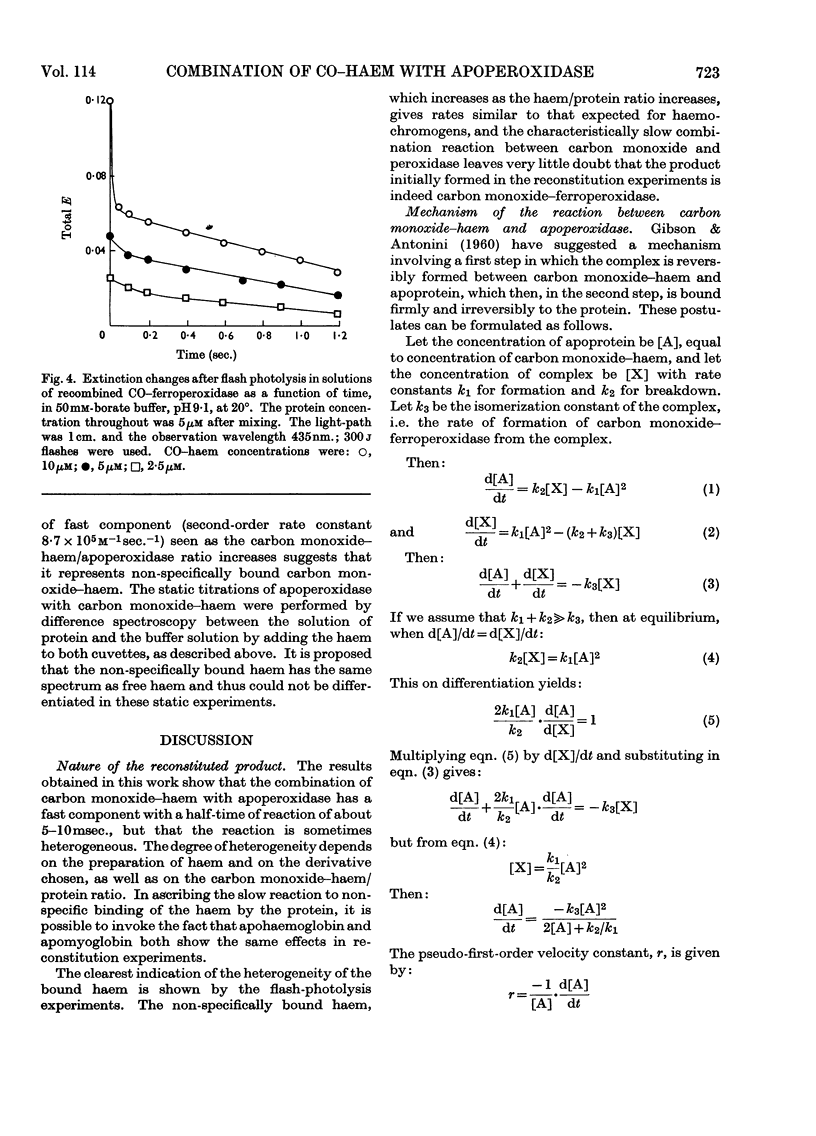
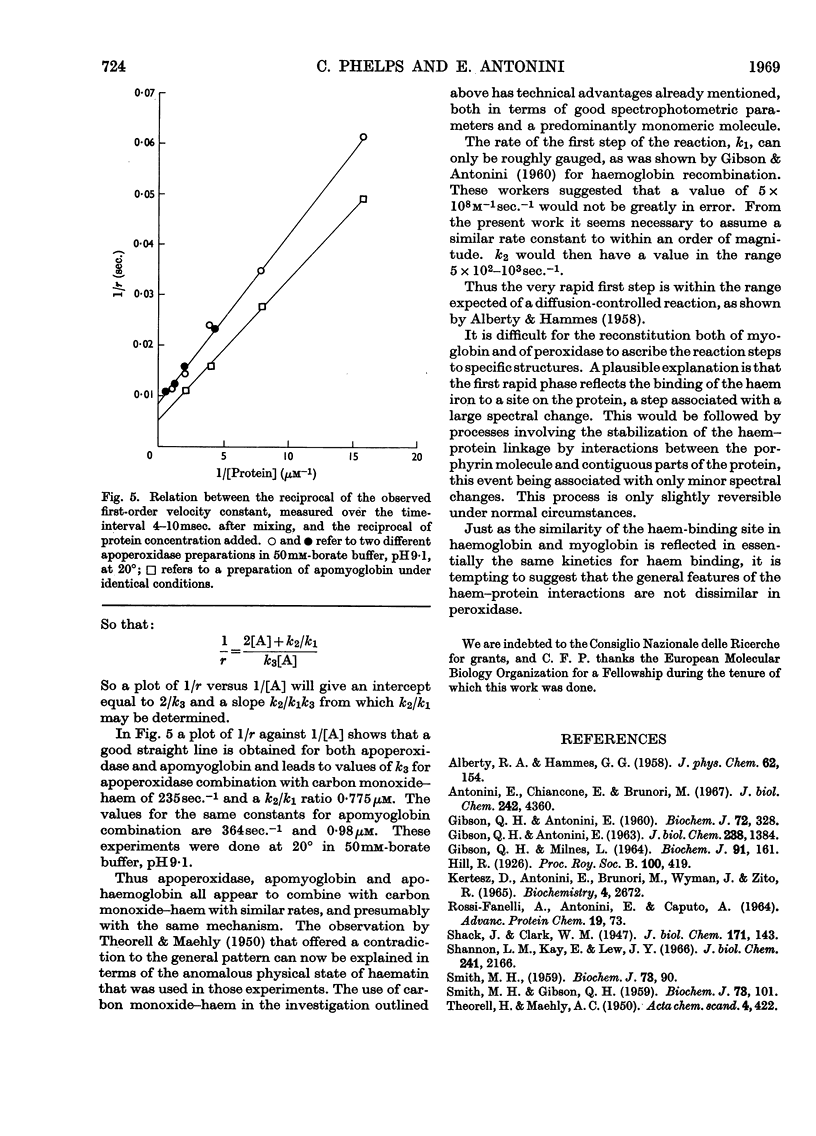
1. Static titrations reveal an exact stoicheiometry between various haem derivatives and apoperoxidase prepared from one isoenzyme of the horseradish enzyme. 2. Carbon monoxide–protohaem reacts rapidly with apoperoxidase and the kinetics can be accounted for by a mechanism already applied to the reaction of carbon monoxide–haem derivatives with apomyoglobin and apohaemoglobin. 3. According to this mechanism a complex is formed first whose combination and dissociation velocity constants are 5×108m−1sec.−1 and 103sec.−1 at pH9·1 and 20°. The complex is converted into carbon monoxide–haemoprotein in a first-order process with a rate constant of 235sec.−1 for peroxidase and 364sec.−1 for myoglobin at pH9·1 and 20°. 4. The effects of pH and temperature were examined. The activation energy for the process of complex-isomerization is about 13kcal./mole. 5. The similarity in the kinetics of the reactions of carbon monoxide–haem with apoperoxidase and with apomyoglobin suggests structural similarities at the haem-binding sites of the two proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Chiancone E., Brunori M. Studies on the relations between molecular and functional properties of hemoglobin. VI. Observations on the kinetics of hemoglobin reactions in concentrated salt solutions. J Biol Chem. 1967 Oct 10;242(19):4360–4366. [PubMed] [Google Scholar]
- GIBSON Q. H., ANTONINI E. Kinetic studies on the reaction between native globin and haem derivatives. Biochem J. 1960 Nov;77:328–341. doi: 10.1042/bj0770328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., ANTONINI E. Rates of reaction of native human globin with some hemes. J Biol Chem. 1963 Apr;238:1384–1388. [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz D., Antonini E., Brunori M., Wyman J., Zito R. Studies on the equilibria and kinetics of the reactions of peroxidases with ligands. I. The reaction of ferroperoxidases with carbon monoxide. Biochemistry. 1965 Dec;4(12):2672–2676. doi: 10.1021/bi00888a016. [DOI] [PubMed] [Google Scholar]
- SMITH M. H., GIBSON Q. H. The preparation and some properties of myoglobin containing meso- and deutero- haem. Biochem J. 1959 Sep;73:101–106. doi: 10.1042/bj0730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon L. M., Kay E., Lew J. Y. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J Biol Chem. 1966 May 10;241(9):2166–2172. [PubMed] [Google Scholar]


