Abstract
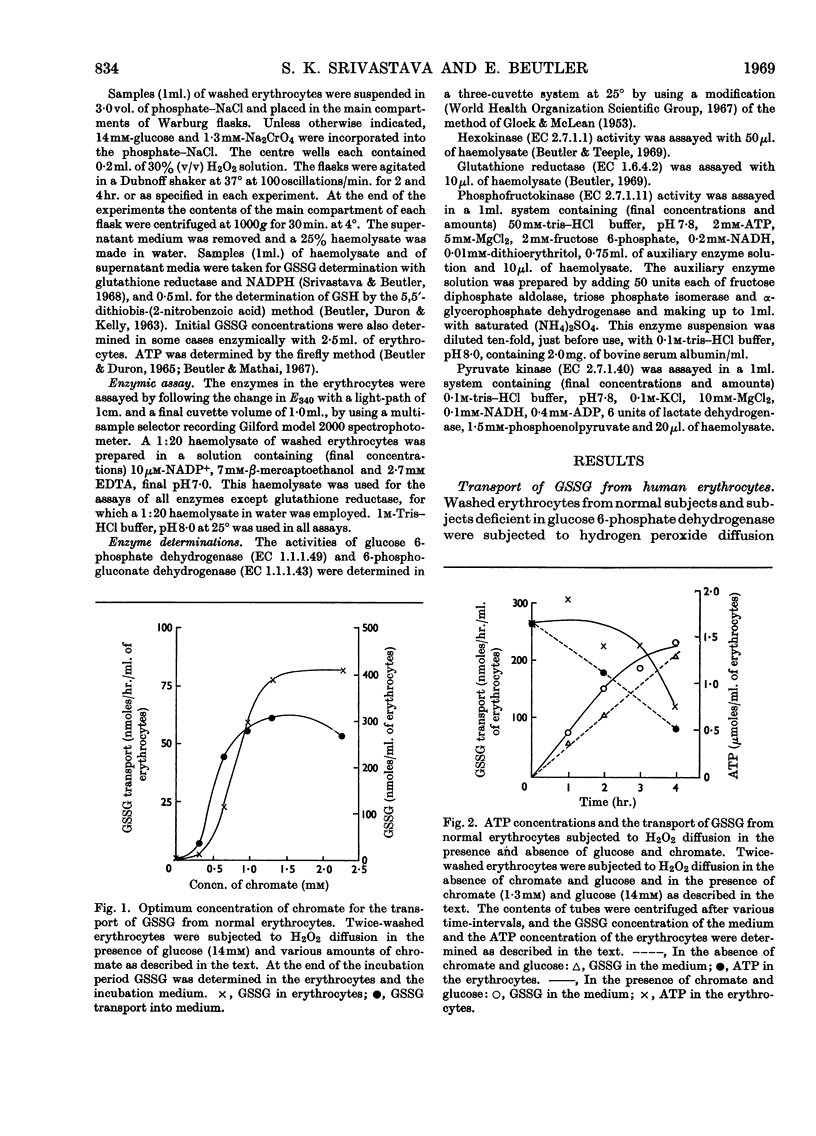
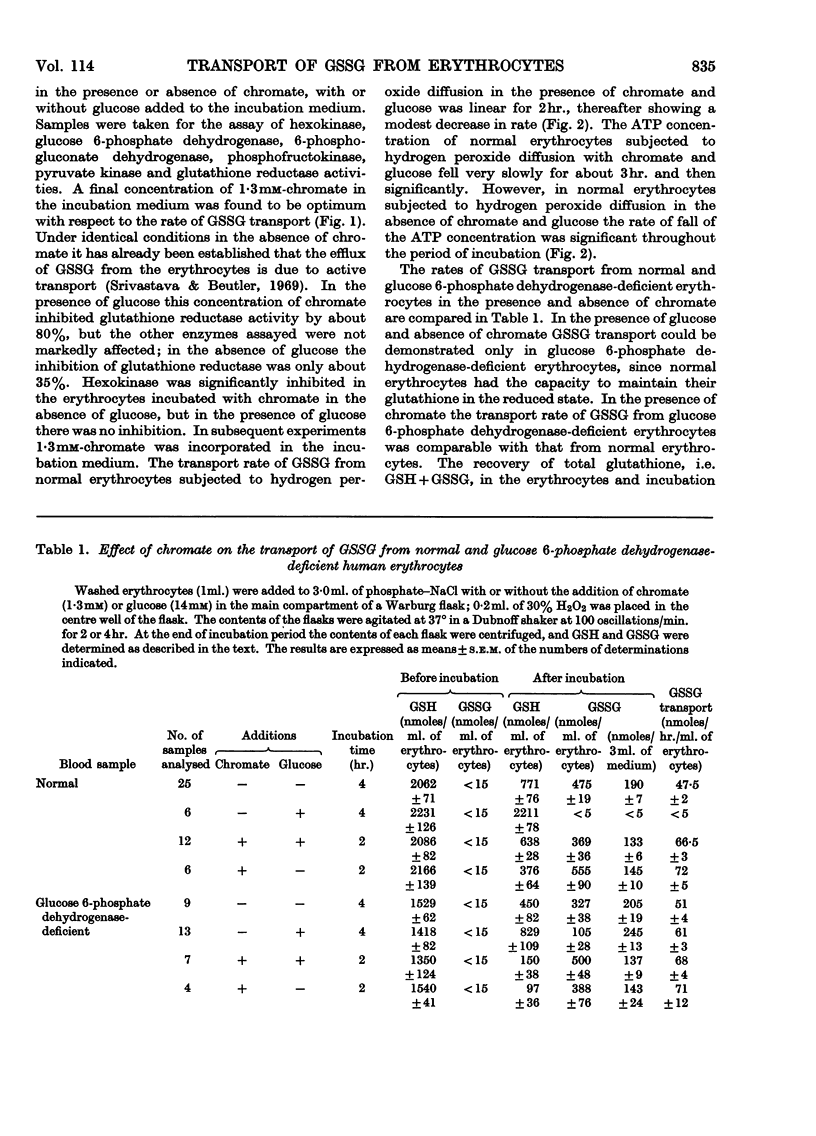
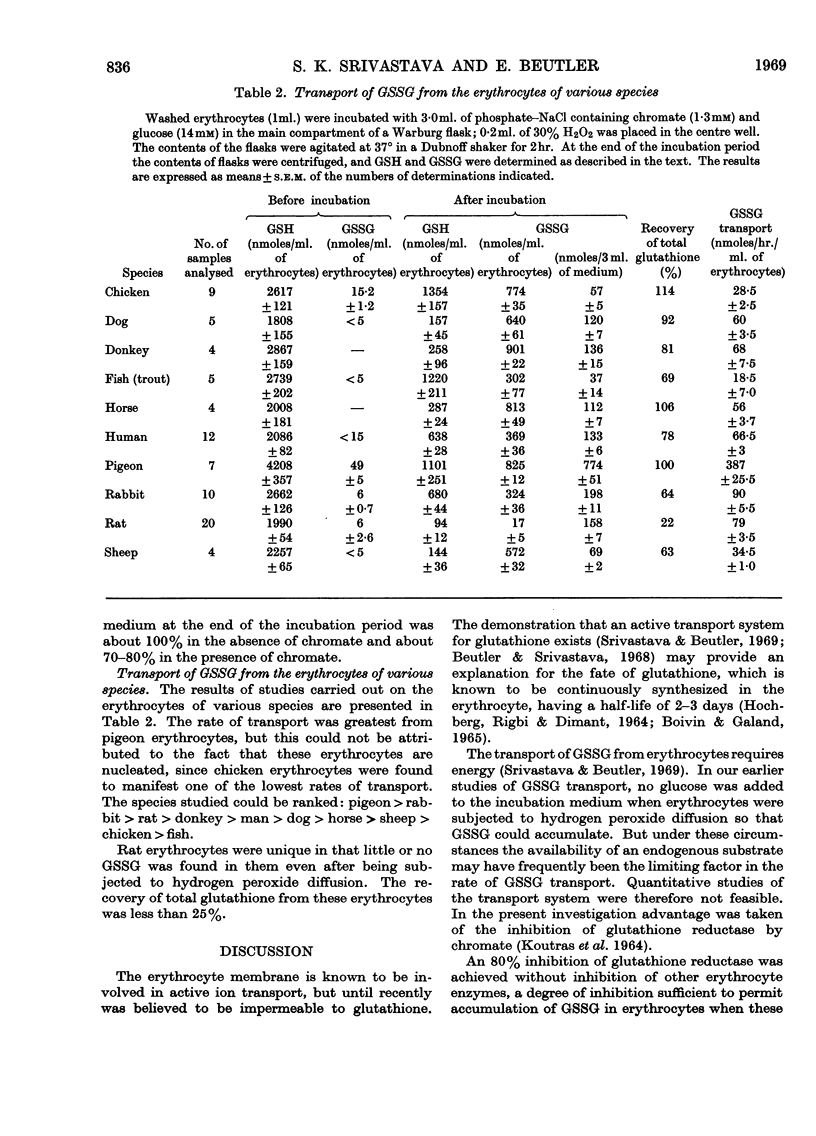
1. Erythrocytes from normal and glucose 6-phosphate dehydrogenase-deficient humans were subjected to hydrogen peroxide diffusion to oxidize the GSH. Studies were carried out in the presence and absence of chromate to inhibit glutathione reductase and with or without the addition of glucose. 2. The GSH content of erythrocytes from other species was oxidized by subjecting them to hydrogen peroxide diffusion in the presence of chromate and glucose. 3. Chromate (1·3mm) inhibited glutathione reductase by about 80%, whereas glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, hexokinase, phosphofructokinase and pyruvate kinase were not inhibited. 4. The GSSG formed was transported from the erythrocytes to the medium. 5. The transport rate of GSSG from glucose 6-phosphate dehydrogenase-deficient erythrocytes subjected to hydrogen peroxide diffusion in the presence of chromate was comparable with that from normal and glucose 6-phosphate dehydrogenase-deficient erythrocytes. 6. The rate of transport of GSSG from erythrocytes of various species studied could be ranked: pigeon>rabbit>rat>donkey>man>dog>horse>sheep>chicken>fish.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- BEUTLER E., DURON O. RED CELL ATP DETERMINATIONS. Blood. 1965 Apr;25:625–625. [PubMed] [Google Scholar]
- Beutler E. Glutathione reductase: stimulation in normal subjects by riboflavin supplementation. Science. 1969 Aug 8;165(3893):613–615. doi: 10.1126/science.165.3893.613. [DOI] [PubMed] [Google Scholar]
- Beutler E., Mathai C. K. A comparison of normal red cell ATP levels as measured by the firefly system and the hexokinase system. Blood. 1967 Sep;30(3):311–320. [PubMed] [Google Scholar]
- Beutler E., Teeple L. Mannose metabolism in the human erythrocyte. J Clin Invest. 1969 Mar;48(3):461–466. doi: 10.1172/JCI106003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOCHBERG A., RIGBI M., DIMANT E. THE INCORPORATION IN VITRO OF GLYCINE AND L-GLUTAMIC ACID INTO GLUTATHIONE OF HUMAN ERYTHROCYTES. Biochim Biophys Acta. 1964 Sep 4;90:464–471. doi: 10.1016/0304-4165(64)90225-9. [DOI] [PubMed] [Google Scholar]
- KOUTRAS G. A., HATTORI M., SCHNEIDER A. S., EBAUGH F. G., Jr, VALENTINE W. N. STUDIES ON CHROMATED ERYTHROCYTES. EFFECT OF SODIUM CHROMATE ON ERYTHROCYTE GLUTATHIONE REDUCTASE. J Clin Invest. 1964 Feb;43:323–331. doi: 10.1172/JCI104917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Accurate measurement of oxidized glutathione content of human, rabbit, and rat red blood cells and tissues. Anal Biochem. 1968 Oct 24;25(1):70–76. doi: 10.1016/0003-2697(68)90082-1. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Permeability of normal and glucose-6-phosphate dehydrogenase deficient erythrocytes to glutathione. Biochem Biophys Res Commun. 1967 Sep 7;28(5):659–664. doi: 10.1016/0006-291x(67)90365-8. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. The transport of oxidized glutathione from human erythrocytes. J Biol Chem. 1969 Jan 10;244(1):9–16. [PubMed] [Google Scholar]


