Abstract
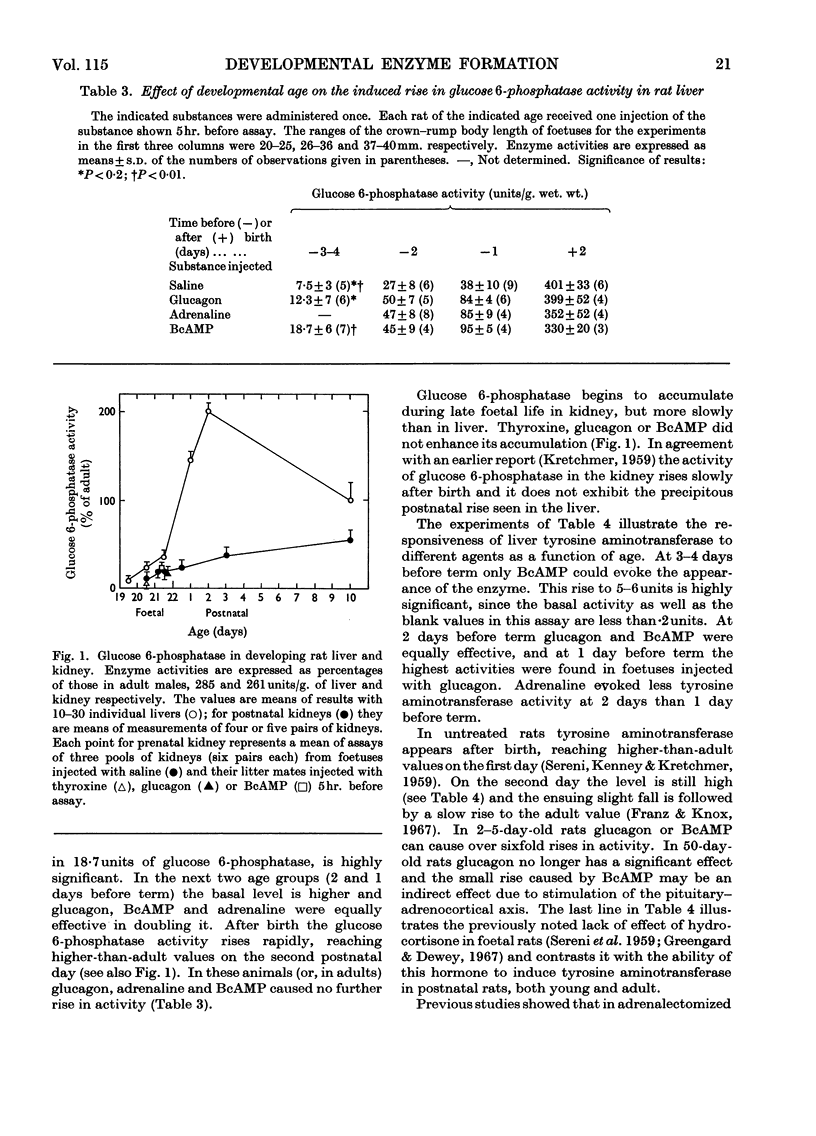
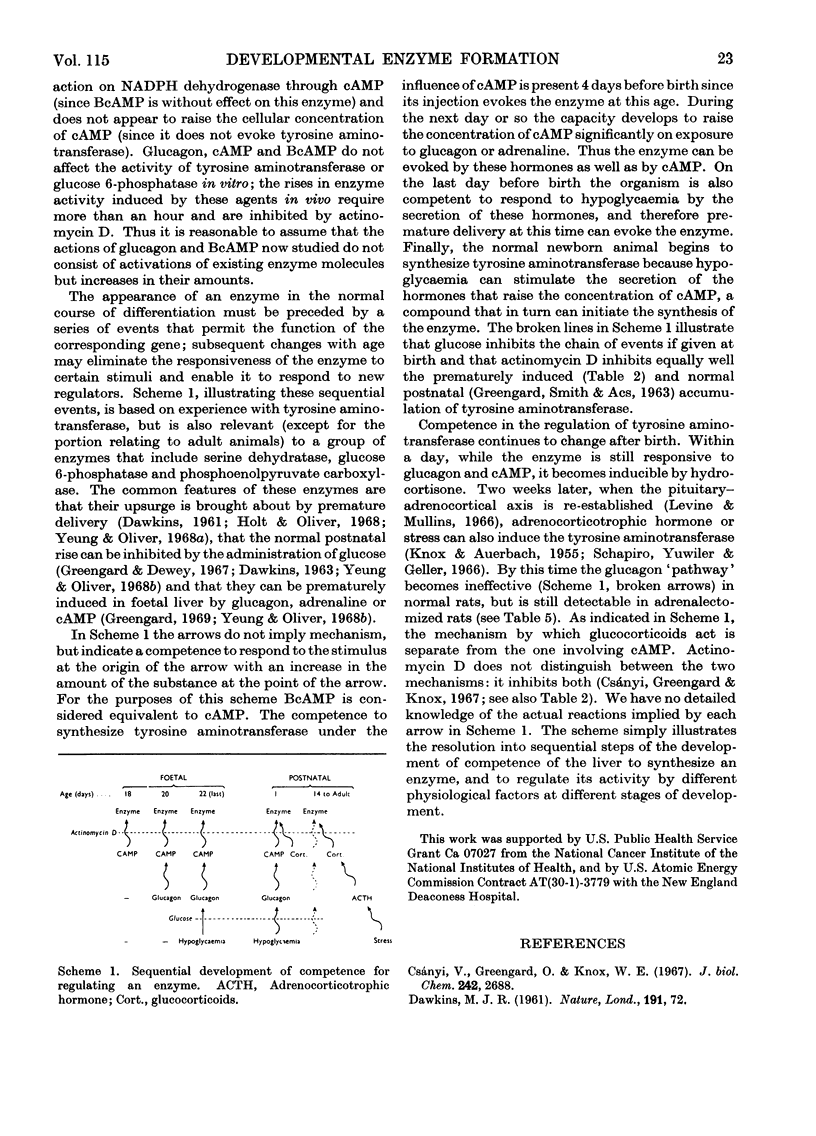
1. The administration of glucagon, cAMP [adenosine 3′,5′-(cyclic)-monophosphate], BcAMP [6-N-2′-O-dibutyryladenosine 3′,5′-(cyclic)-monophosphate] or adrenaline to foetal rats during the last 2 days of gestation evoked the appearance of tyrosine aminotransferase and enhanced the accumulation of glucose 6-phosphatase in the liver. In foetuses 1–2 days younger only BcAMP was effective. After birth liver glucose 6-phosphatase no longer responds to glucagon or BcAMP. Tyrosine aminotransferase is still inducible by these agents in 2-day-old rats, but not in 50-day-old rats. After adrenalectomy of adults glucagon or BcAMP can enhance the induction of the enzyme by hydrocortisone. The results indicate that the ability to synthesize tyrosine aminotransferase and glucose 6-phosphatase when exposed to cAMP develops sooner than the ability to respond to glucagon with an increase in the concentration of cAMP; the responsiveness of enzymes to different hormones changes with age. A scheme illustrating the sequential development of competence in regulating the level of an enzyme is presented. 2. Actinomycin inhibited the effects of glucagon and BcAMP on liver tyrosine aminotransferase and glucose 6-phosphatase in foetal rats. Growth hormone, insulin and hydrocortisone did not enhance the formation of these enzymes. 3. The time-course of accumulation of glucose 6-phosphatase in the kidney is different from that in the liver. Hormones that increase the accumulation in foetal liver do not do so in the kidney of the same foetus or in the livers of postnatal rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAWKINS M. J. Changes in glucose-6-phosphatase activity in liver and kidney at birth. Nature. 1961 Jul 1;191:72–73. doi: 10.1038/191072b0. [DOI] [PubMed] [Google Scholar]
- DAWKINS M. J. GLYCOGEN SYNTHESIS AND BREAKDOWN IN FETAL AND NEWBORN RAT LIVER. Ann N Y Acad Sci. 1963 Dec 30;111:203–211. doi: 10.1111/j.1749-6632.1963.tb36960.x. [DOI] [PubMed] [Google Scholar]
- Franz J. M., Knox W. E. The effect of development and hydrocortisone on tryptophan oxygenase, formamidase, and tyrosine aminotransferase in the livers of young rats. Biochemistry. 1967 Nov;6(11):3464–3471. doi: 10.1021/bi00863a017. [DOI] [PubMed] [Google Scholar]
- GREENGARD O., SMITH M. A., ACS G. Relation of cortisone and synthesis of ribonucleic acid to induced and developmental enzyme formation. J Biol Chem. 1963 Apr;238:1548–1551. [PubMed] [Google Scholar]
- Greengard O., Baker G. T. Glucagon, starvation, and the induction of liver enzymes by hydrocortisone. Science. 1966 Dec 16;154(3755):1461–1462. [PubMed] [Google Scholar]
- Greengard O., Dewey H. K. Initiation by glucagon of the premature development of tyrosine aminotransferase, serine dehydratase, and glucose-6-phosphatase in fetal rat liver. J Biol Chem. 1967 Jun 25;242(12):2986–2991. [PubMed] [Google Scholar]
- Greengard O., Dewey H. K. The developmental formation of liver glucose 6-phosphatase and reduced nicotinamide adenine dinucleotide phosphate dehydrogenase in fetal rats treated with thyroxine. J Biol Chem. 1968 May 25;243(10):2745–2749. [PubMed] [Google Scholar]
- Greengard O. Enzymic differentiation in mammalian liver injection of fetal rats with hormones causes the premature formation of liver enzymes. Science. 1969 Feb 28;163(3870):891–895. doi: 10.1126/science.163.3870.891. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Oliver I. T. Factors affecting the premature induction of tyrosine aminotransferase in foetal rat liver. Biochem J. 1968 Jun;108(2):333–338. doi: 10.1042/bj1080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOX W. E., AUERBACH V. H. The hormonal control of tryptophan peroxidase in the rat. J Biol Chem. 1955 May;214(1):307–313. [PubMed] [Google Scholar]
- KRETCHMER N. Enzymatic patterns during development; an approach to a biochemical definition of immaturity. Pediatrics. 1959 Mar;23(3):606–617. [PubMed] [Google Scholar]
- Levine S., Mullins R. F., Jr Hormonal influences on brain organization in infant rats. Science. 1966 Jun 17;152(3729):1585–1592. doi: 10.1126/science.152.3729.1585. [DOI] [PubMed] [Google Scholar]
- POSTERNAK T., SUTHERLAND E. W., HENION W. F. Derivatives of cyclic 3',5'-adenosine monophosphate. Biochim Biophys Acta. 1962 Dec 17;65:558–560. doi: 10.1016/0006-3002(62)90475-4. [DOI] [PubMed] [Google Scholar]
- Reshef L., Greengard O. The effect of amino acid mixtures, insulin, epinephrine and glucagon in vivo on the levels of rat liver tyrosine aminotransferase. Enzymol Biol Clin (Basel) 1969;10(2):113–121. doi: 10.1159/000458302. [DOI] [PubMed] [Google Scholar]
- SERENI F., KENNEY F. T., KRETCHMER N. Factors influencing the development of tyrosine-alpha-ketoglutarate transaminase activity in rat liver. J Biol Chem. 1959 Mar;234(3):609–612. [PubMed] [Google Scholar]
- Schapiro S., Yuwiler A., Geller E. Maturation of a stress-activated mechanism inhibiting induction of tyrosine transaminase. Science. 1966 Jun 17;152(3729):1642–1643. doi: 10.1126/science.152.3729.1642. [DOI] [PubMed] [Google Scholar]
- Wicks W. D. Induction of tyrosine-alpha-ketoglutarate transaminase in fetal rat liver. J Biol Chem. 1968 Mar 10;243(5):900–906. [PubMed] [Google Scholar]
- Wicks W. D. Tyrosine-alpha-ketoglutarate transaminase: induction by epinephrine and adenosine-3',5'-cyclic phosphate. Science. 1968 May 31;160(3831):997–998. doi: 10.1126/science.160.3831.997. [DOI] [PubMed] [Google Scholar]
- Yeung D., Oliver I. T. Factors affecting the premature induction of phosphopyruvate carboxylase in neonatal rat liver. Biochem J. 1968 Jun;108(2):325–331. doi: 10.1042/bj1080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung D., Oliver I. T. Induction of phosphopyruvate carboxylase in neonatal rat liver by adenosine 3',5'-cyclic monophosphate. Biochemistry. 1968 Sep;7(9):3231–3239. doi: 10.1021/bi00849a028. [DOI] [PubMed] [Google Scholar]


