Abstract
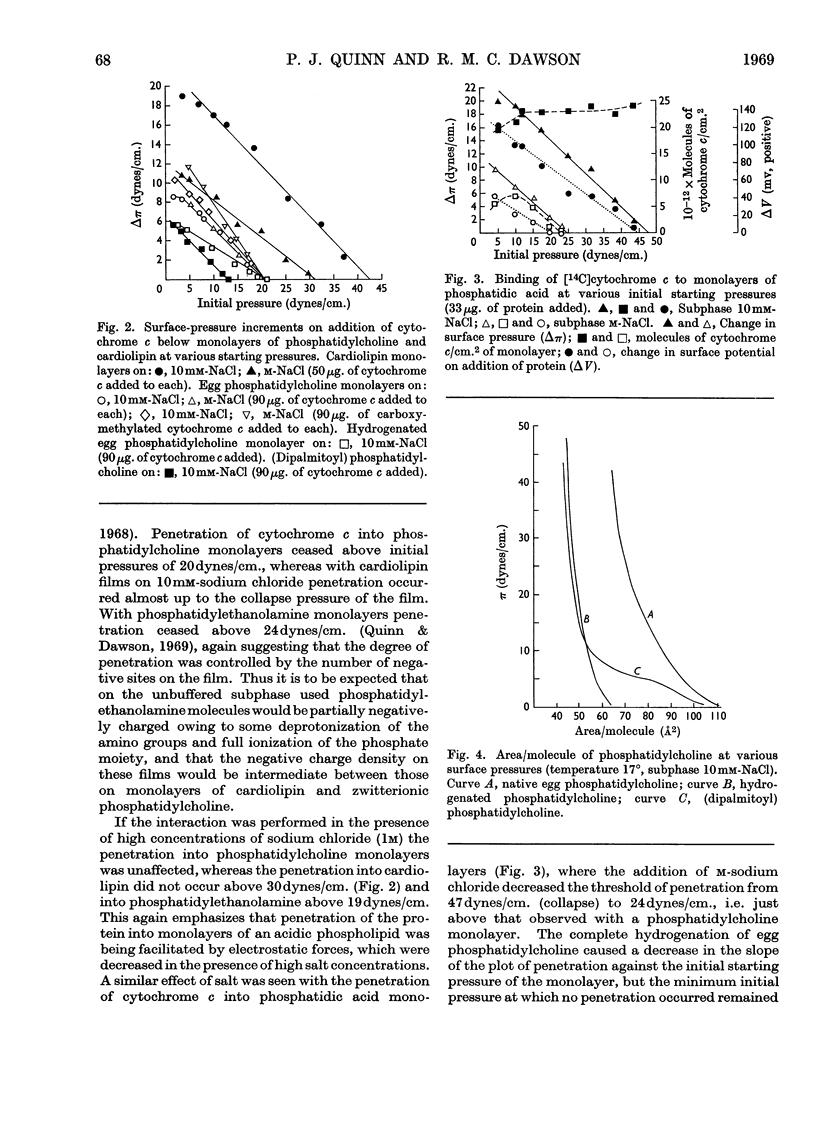
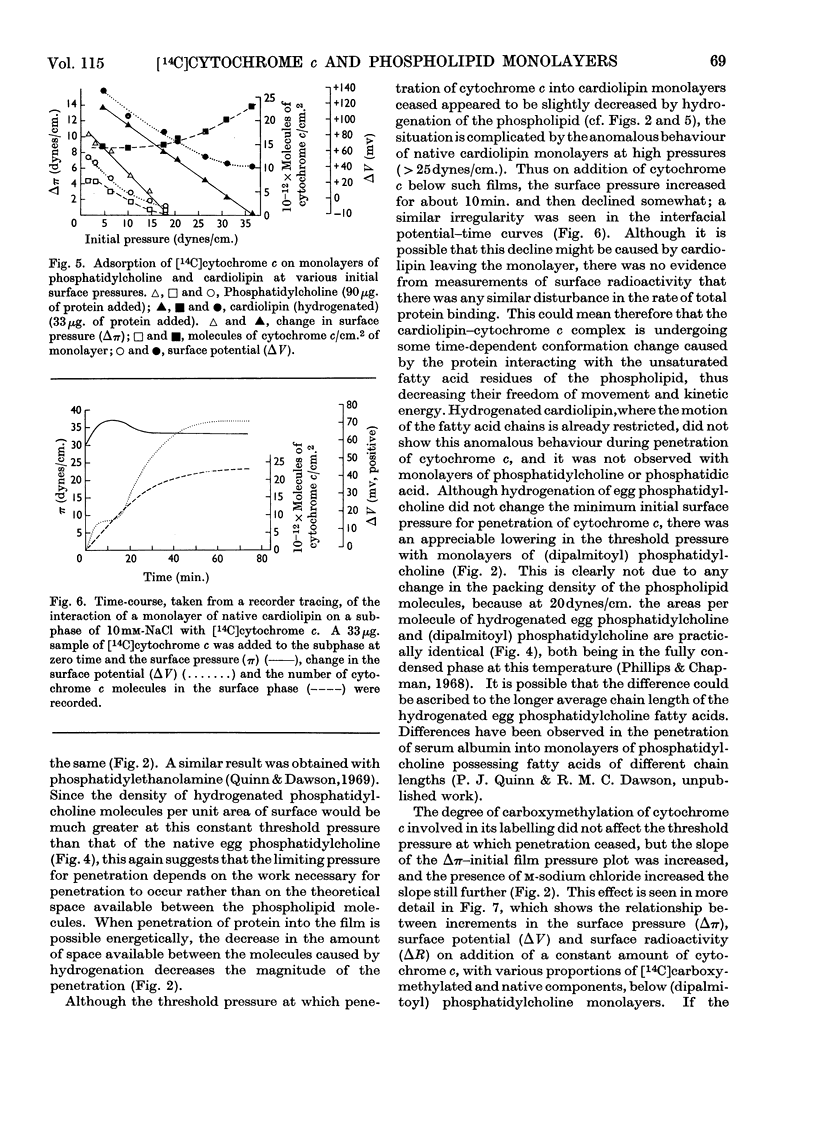
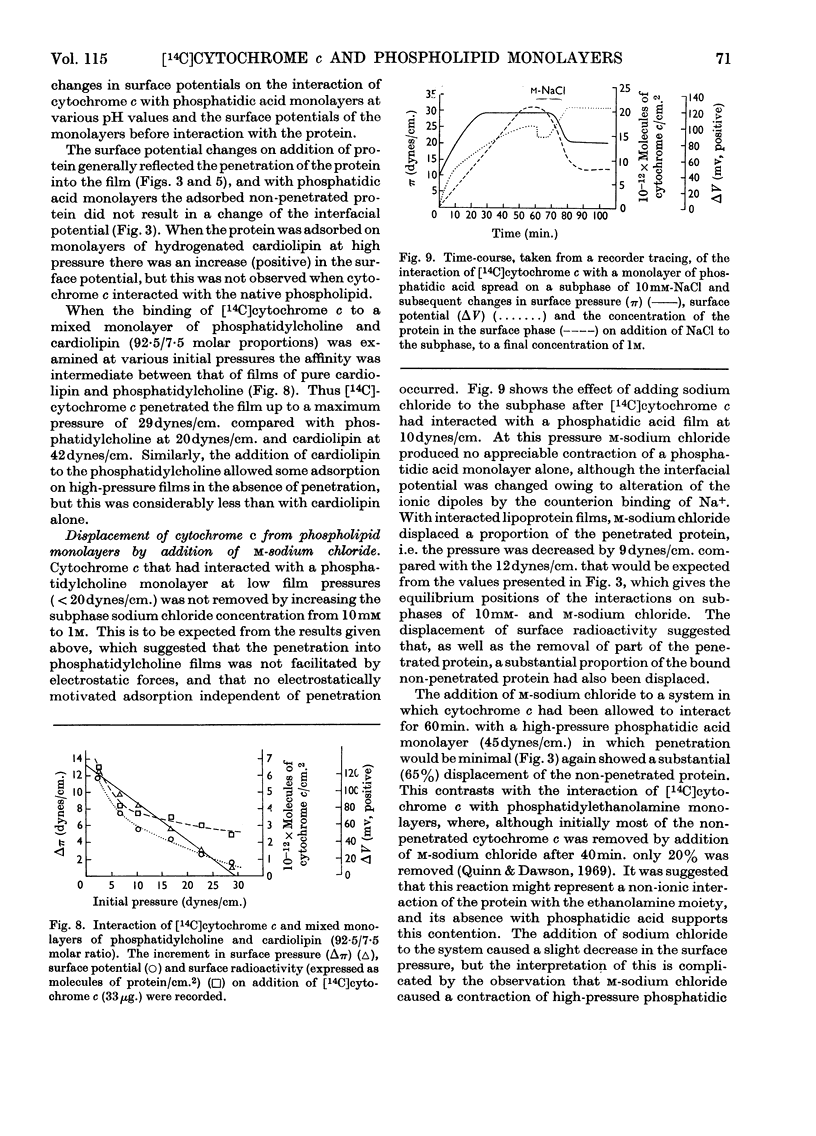
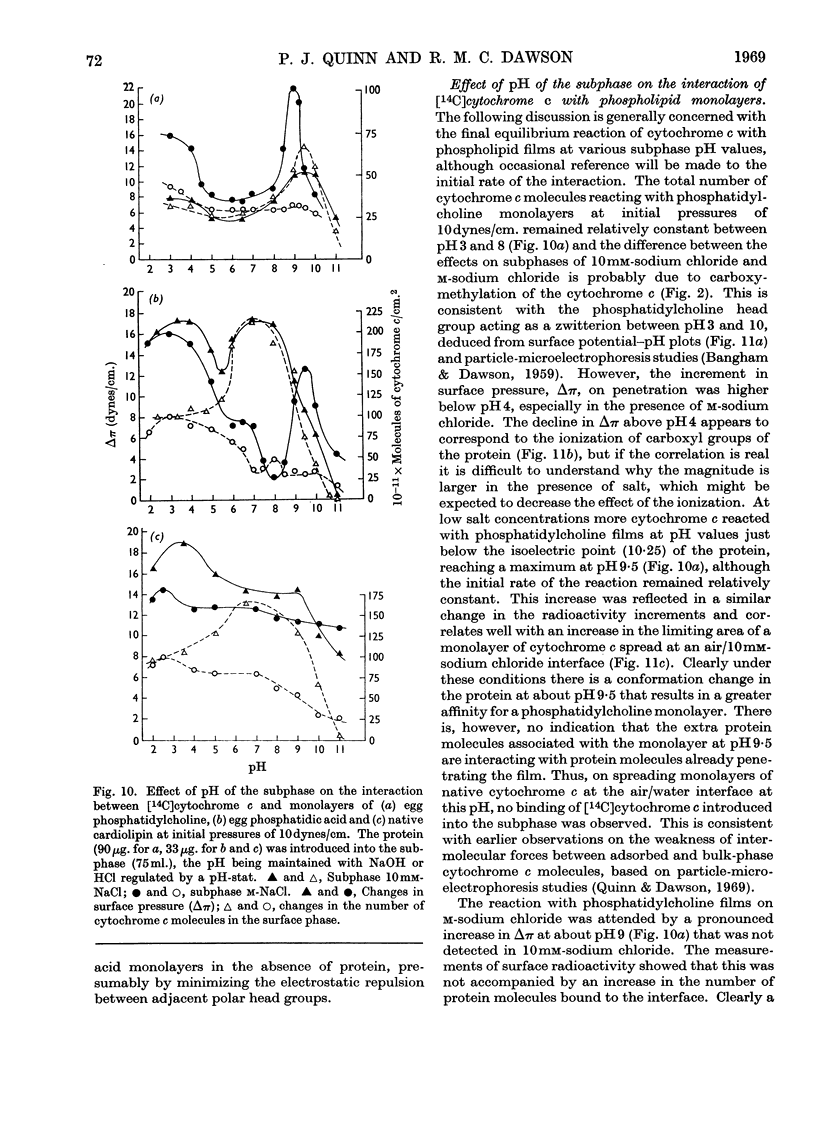
1. The interactions between cytochrome c (native and [14C]carboxymethylated) and monolayers of phosphatidylcholine, phosphatidic acid and cardiolipin at the air/water interface was investigated by measurements of surface radioactivity, pressure and potential. 2. On a subphase of 10mm-or m-sodium chloride, penetration of cytochrome c into egg phosphatidylcholine monolayers, as measured by an increase of surface pressure, and the number of molecules penetrating, as judged by surface radioactivity, were inversely proportional to the initial pressure of the monolayer and became zero at 20dynes/cm. The constant of proportionality was increased when the cytochrome c was carboxymethylated or decreased when the phospholipid was hydrogenated, but the cut-off point remained at 20dynes/cm. 3. Penetrated cytochrome c could be removed almost entirely by compression of the phosphatidylcholine monolayer above 20dynes/cm. 4. With phosphatidic acid and cardiolipin monolayers on 10mm-sodium chloride the binding of cytochrome c was much stronger and cytochrome c penetrated into films nearing the collapse pressure (>40dynes/cm.). The penetration was partly electrostatically facilitated, since it was decreased by carrying out the reaction on a subphase of m-sodium chloride, and the relationship between the surface pressure increment and the initial film pressure moved nearer to that observed with phosphatidylcholine. 5. Surface radioactivity determinations showed that [14C]carboxymethylated cytochrome c was still adsorbed on phosphatidic acid and cardiolipin monolayers after the cessation of penetration. This adsorption was primarily electrostatic in nature because it could be prevented and substantially reversed by adding m-sodium chloride to the subphase and there was no similar adsorption on phosphatidylcholine films. 6. The penetration into and adsorption on the three phospholipid monolayers was examined as a function of the pH of the subphase and compared with the state of ionization of both the phospholipid and the protein, and the area occupied by the latter at an air/water interface. 7. It is concluded that the binding of cytochrome c to phospholipids can only be partially understood by a consideration of the ionic interaction between the components and that subtle conformational changes in the protein must affect the magnitude and stability of the complex. 8. If cytochrome c is associated with a phospholipid in mitochondria then cardiolipin would fulfil the characteristics of the binding most adequately.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVAILABILITY of fluorides. Nutr Rev. 1959 Jan;17(1):29–30. doi: 10.1111/j.1753-4887.1959.tb06373.x. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D., DAWSON R. M. The relation between the activity of a lecithinase and the electrophoretic charge of the substrate. Biochem J. 1959 Jul;72:486–492. doi: 10.1042/bj0720486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camejo G., Colacicco G., Rapport M. M. Lipid monolayers: interactions with the apoprotein of high density plasma lipoprotein. J Lipid Res. 1968 Sep;9(5):562–569. [PubMed] [Google Scholar]
- DAWSON R. M., HEMINGTON N., DAVENPORT J. B. Improvements in the method of determining individual phospholipids in a complex mixture by successive chemical hydrolyses. Biochem J. 1962 Sep;84:497–501. doi: 10.1042/bj0840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. ON THE MECHANISM OF ACTION OF PHOSPHOLIPASE A. Biochem J. 1963 Sep;88:414–423. doi: 10.1042/bj0880414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. L., Criddle R. S. Binding of cytochrome c by mitochondrial structural protein. Biochemistry. 1966 Feb;5(2):583–588. doi: 10.1021/bi00866a026. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Getz G. S., Bartley W., Lurie D., Notton B. M. The phospholipids of various sheep organs, rat liver and of their subcellular fractions. Biochim Biophys Acta. 1968 Mar 4;152(2):325–339. doi: 10.1016/0005-2760(68)90040-4. [DOI] [PubMed] [Google Scholar]
- González-Cadavid N. F., Campbell P. N. Subcellular distribution of cytochrome c in rat liver. Methods for its extraction and purification. Biochem J. 1967 Nov;105(2):427–442. doi: 10.1042/bj1050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Dawson R. M. The binding of calcium at lipid-water interfaces. Eur J Biochem. 1967 Mar;1(1):61–69. doi: 10.1007/978-3-662-25813-2_11. [DOI] [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. The reversible removal of cytochrome c from mitochondria. J Biol Chem. 1960 Feb;235:531–534. [PubMed] [Google Scholar]
- MacLennan D. H., Lenaz G., Szarkowska L. Studies on the mechanims of oxidative phosphorylation. IX. Effect of cytochrome c on energy-linked processes. J Biol Chem. 1966 Nov 25;241(22):5251–5259. [PubMed] [Google Scholar]
- Margoliash E., Schejter A. Cytochrome c. Adv Protein Chem. 1966;21:113–286. doi: 10.1016/s0065-3233(08)60128-x. [DOI] [PubMed] [Google Scholar]
- Mirsky R., George P. Optical rotatory dispersion and spectral properties of yeast isocytochromes c. Biochemistry. 1967 Dec;6(12):3671–3675. doi: 10.1021/bi00864a008. [DOI] [PubMed] [Google Scholar]
- Mirsky R., George P. Optical rotatory dispersion spectra of cytochrome C and polymers. Proc Natl Acad Sci U S A. 1966 Jul;56(1):222–229. doi: 10.1073/pnas.56.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer F. B., Dawson R. M. Complex-formation between triphosphoinositide and experimental allergic encephalitogenic protein. Biochem J. 1969 Mar;111(5):637–645. [PubMed] [Google Scholar]
- Phillips M. C., Chapman D. Monolayer characteristics of saturated 1,2,-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface. Biochim Biophys Acta. 1968 Nov 5;163(3):301–313. doi: 10.1016/0005-2736(68)90115-6. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. The hydrolysis of monolayers of phosphatidyl(Me-14C)choline by phospholipase D. Biochem J. 1969 Jul;113(4):697–705. doi: 10.1042/bj1130697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J., Dawson R. M. The interaction of cytochrome c with monolayers of phosphatidylethanolamine. Biochem J. 1969 Aug;113(5):791–803. doi: 10.1042/bj1130791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley G. G., Leslie R. B., Chapman D. Small-angle x-ray scattering studies of cytochrome c-phospholipid complexes. Biochim Biophys Acta. 1969 Jan 28;173(1):1–10. doi: 10.1016/0005-2736(69)90031-5. [DOI] [PubMed] [Google Scholar]
- Shipley G. G., Leslie R. B., Chapman D. X-ray diffraction study of the interaction of phospholipids with cytochrome c in the aqueous phase. Nature. 1969 May 10;222(5193):561–562. doi: 10.1038/222561a0. [DOI] [PubMed] [Google Scholar]
- Snart R. S., Sanyal N. N. Interaction of polypeptide hormones with lipid monolayers. Biochem J. 1968 Jul;108(3):369–373. doi: 10.1042/bj1080369. [DOI] [PMC free article] [PubMed] [Google Scholar]


