Abstract
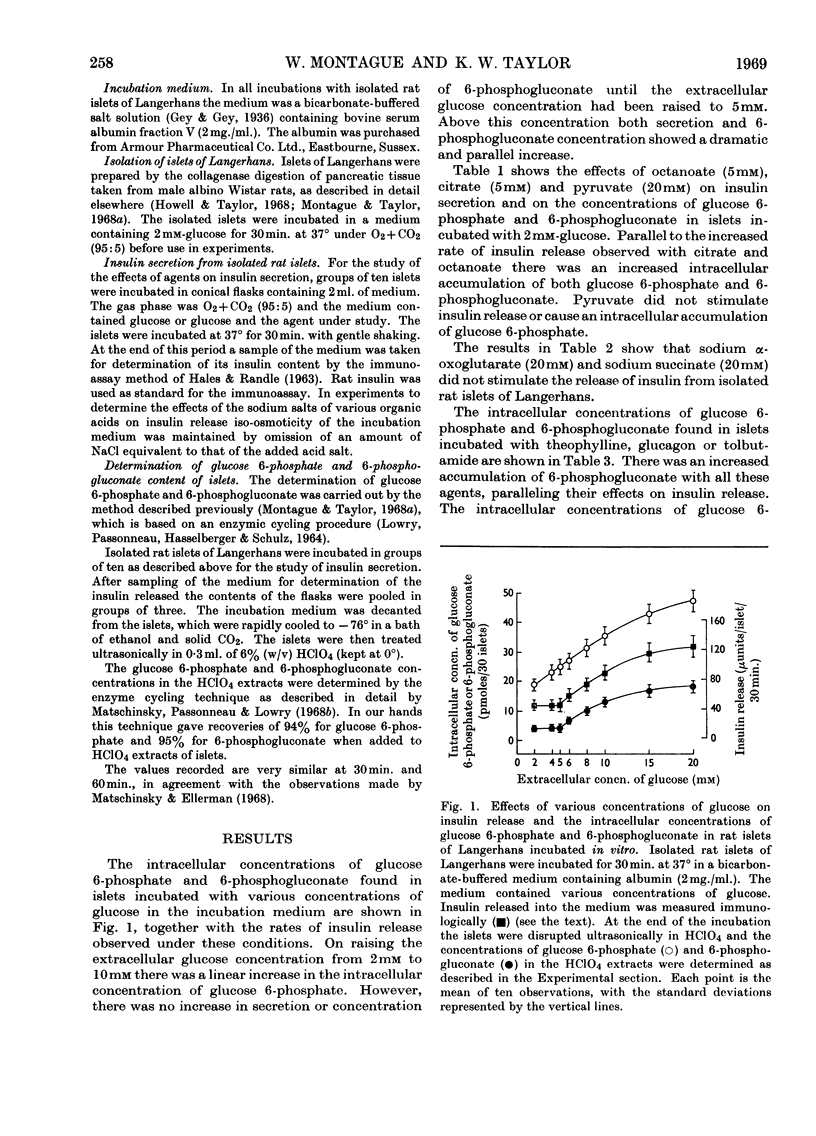
1. Concentrations of glucose 6-phosphate and 6-phosphogluconate were studied in islets of Langerhans isolated from rat pancreas and incubated in the presence of various agents that induce insulin release. 2. In response to rising concentrations of extracellular glucose (2–10mm) there is a linear increase in the intracellular concentration of glucose 6-phosphate, though this is not the case for 6-phosphogluconate, the intracellular concentration of which only increases when the external glucose concentration exceeds 5mm. 3. Tolbutamide, octanoate and citrate, all of which promote insulin secretion from isolated islets, increase the intracellular concentrations of glucose 6-phosphate and 6-phosphogluconate. The results obtained in the presence of octanoate and citrate are compatible with an inhibitory effect of citrate on islet-cell phosphofructokinase. 4. Theophylline and glucagon when incubated with islets in vitro promote insulin release and cause a rise in 6-phosphogluconate concentration and not in that of glucose 6-phosphate. 5. It is suggested that the further metabolism of glucose 6-phosphate through a pathway other than glycolysis is essential for insulin release. One such pathway involves its oxidation to 6-phosphogluconate, which seems to be a necessary accompaniment of insulin secretion due to glucose. The possibility that agents other than glucose promote insulin release by enhancing the oxidation of glucose 6-phosphate through this pathway is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Randle P. J. Glucose phosphorylation in mouse pancreatic islets. Biochem J. 1968 Apr;107(4):599–600. doi: 10.1042/bj1070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Randle P. J. Glucose-6-phosphatase activity of mouse pancreatic islets. Nature. 1968 Aug 24;219(5156):857–858. doi: 10.1038/219857a0. [DOI] [PubMed] [Google Scholar]
- Brolin S. E., Berne C., Linde B. Measurements of the enzymatic activities required for ATP formation by glycolysis in the pancreatic islets of hyperglycemic mice (NZO). Diabetes. 1967 Jan;16(1):21–25. doi: 10.2337/diab.16.1.21. [DOI] [PubMed] [Google Scholar]
- Brolin S. E., Berne C., Petersson B., Larsson A. Histochemical staining and microchemical studies of fructose 1,6-diphosphate splitting enzymes in the pancreatic islets and liver of NZO mice. J Histochem Cytochem. 1968 Oct;16(10):654–658. doi: 10.1177/16.10.654. [DOI] [PubMed] [Google Scholar]
- Butcher R. W. Cyclic 3',5'-AMP and the lipolytic effects of hormones on adipose tissue. Pharmacol Rev. 1966 Mar;18(1):237–241. [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRODSKY G. M., BATTS A. A., BENNETT L. L., VCELLA C., MCWILLIAMS N. B., SMITH D. F. EFFECTS OF CARBOHYDRATES ON SECRETION OF INSULIN FROM ISOLATED RAT PANCREAS. Am J Physiol. 1963 Oct;205:638–644. doi: 10.1152/ajplegacy.1963.205.4.638. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Potassium ions and the secretion of insulin by islets of Langerhans incubated in vitro. Biochem J. 1968 Jun;108(1):17–24. doi: 10.1042/bj1080017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idahl L. A., Hellman B. Microchemical assays of glucose and glucose-6-phosphate in mammalian pancreatic beta-cells. Acta Endocrinol (Copenh) 1968 Nov;59(3):479–486. doi: 10.1530/acta.0.0590479. [DOI] [PubMed] [Google Scholar]
- Krass M. E., LaBella F. S. Oxidation of 14-C-1 and 14-C-6-glucose by hormone synthesizing and hormone secreting portions of neurohypophysial neurons. Mol Pharmacol. 1965 Nov;1(3):306–311. [PubMed] [Google Scholar]
- Kuzuya T., Kanazawa Y., Kosaka K. Stimulation of insulin secretion by xylitol in dogs. Endocrinology. 1969 Feb;84(2):200–207. doi: 10.1210/endo-84-2-200. [DOI] [PubMed] [Google Scholar]
- LACY P. E. Electron microscopy of the beta cell of the pancreas. Am J Med. 1961 Dec;31:851–859. doi: 10.1016/0002-9343(61)90024-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Mayhew D. A possible role for the adenylcyclase system in insulin secretion. J Clin Invest. 1967 Nov;46(11):1724–1734. doi: 10.1172/JCI105663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., King S. Effects of neutral red and imidazole upon insulin secretion. Diabetologia. 1968 Dec;4(6):370–374. doi: 10.1007/BF01211774. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Wright P. H. A new method for the measurement in vitro of pancreatic insulin secretion. Endocrinology. 1967 Jan;80(1):99–108. doi: 10.1210/endo-80-1-99. [DOI] [PubMed] [Google Scholar]
- Martin J. M., Gagliardino J. J. Effect of growth hormone on the isolated pancreatic islets of rat in vitro. Nature. 1967 Feb 11;213(5076):630–631. doi: 10.1038/213630a0. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968 May 25;243(10):2730–2736. [PubMed] [Google Scholar]
- Matschinsky F. M., Kauffman F. C., Ellerman J. E. Effect of hyperglycemia on the hexose monophosphate shunt in islets of Langerhans. Diabetes. 1968 Aug;17(8):475–480. doi: 10.2337/diab.17.8.475. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Passonneau J. V., Lowry O. H. Quantitative histochemical analysis of glycolytic intermediates and cofactors with an oil well technique. J Histochem Cytochem. 1968 Jan;16(1):29–39. doi: 10.1177/16.1.29. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Rutherford C. R., Ellerman J. E. Accumulation of citrate in pancreatic islets of obese hyperglycemic mice. Biochem Biophys Res Commun. 1968 Dec 9;33(5):855–862. doi: 10.1016/0006-291x(68)90240-4. [DOI] [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Pentitols and insulin release by isolated rat islets of Langerhans. Biochem J. 1968 Sep;109(3):333–339. doi: 10.1042/bj1090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Regulation of insulin secretion by short chain fatty acids. Nature. 1968 Mar 2;217(5131):853–853. doi: 10.1038/217853a0. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Ashcroft S. J. Carbohydrate metabolism in pancreatic islets and the release of insulin. Biochem J. 1969 Mar;112(1):1P–2P. doi: 10.1042/bj1120001p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanbar S. S., Martin J. M. Stimulation by octanoate of insulin release from isolated rat pancreas. Metabolism. 1967 May;16(5):482–484. doi: 10.1016/0026-0495(67)90140-0. [DOI] [PubMed] [Google Scholar]
- Sloviter H. A., Petkovic M. R. Stimulation of insulin secretion in the rabbit by D-ribose. Nature. 1969 Jan 25;221(5178):371–372. doi: 10.1038/221371a0. [DOI] [PubMed] [Google Scholar]
- Sussman K. E., Vaughan G. D. Insulin release after ACTH, glucagon and adenosine-3'-5'-phosphate (cyclic AMP) in the perfused isolated rat pancreas. Diabetes. 1967 Jul;16(7):449–454. doi: 10.2337/diab.16.7.449. [DOI] [PubMed] [Google Scholar]
- Turtle J. R., Kipnis D. M. An adrenergic receptor mechanism for the control of cyclic 3'5' adenosine monophosphate synthesis in tissues. Biochem Biophys Res Commun. 1967 Sep 7;28(5):797–802. doi: 10.1016/0006-291x(67)90388-9. [DOI] [PubMed] [Google Scholar]
- WEAVER G., LANDAU B. R. CONTRIBUTION OF THE PENTOSE CYCLE TO GLUCOSE METABOLISM BY ADRENAL CORTEX IN VITRO. Endocrinology. 1963 Nov;73:640–646. doi: 10.1210/endo-73-5-640. [DOI] [PubMed] [Google Scholar]
- Watkins D., Cooperstein S. J., Dixit P. K., Lazarow A. Insulin secretion from toadfish islet tissue stimulated by pyridine nucleotides. Science. 1968 Oct 11;162(3850):283–284. doi: 10.1126/science.162.3850.283. [DOI] [PubMed] [Google Scholar]


