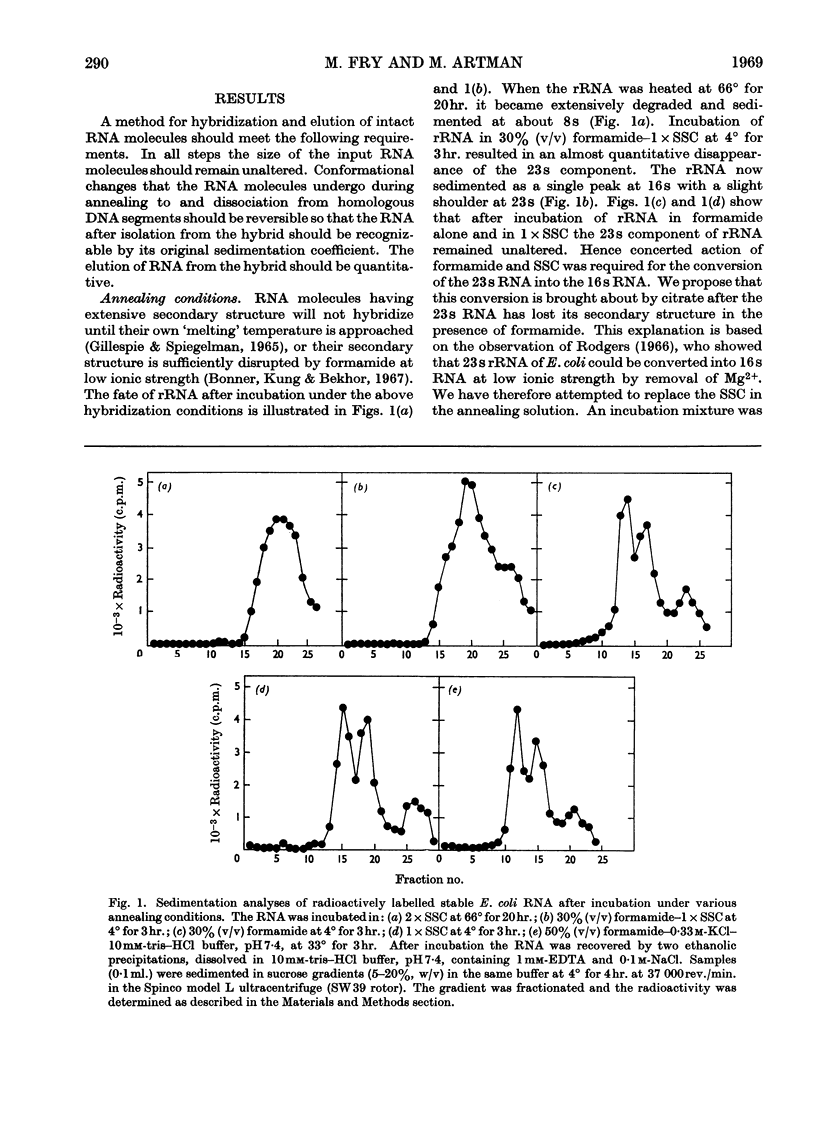
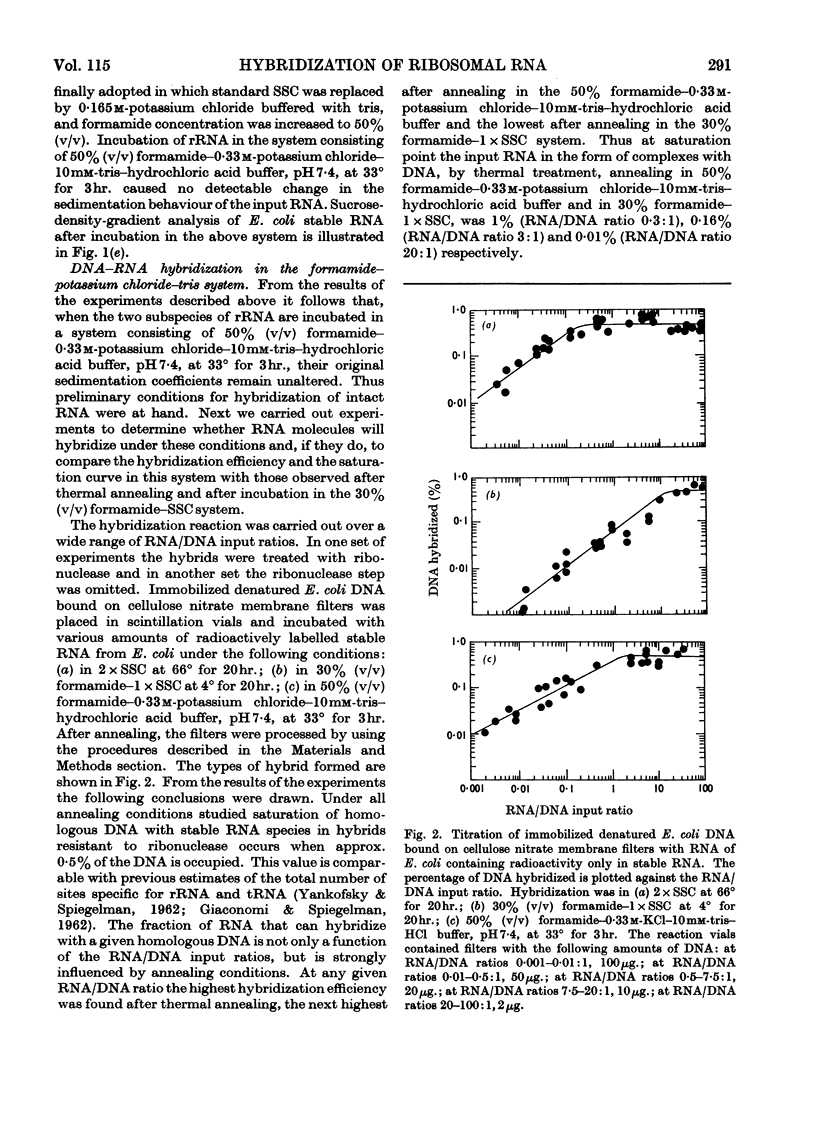
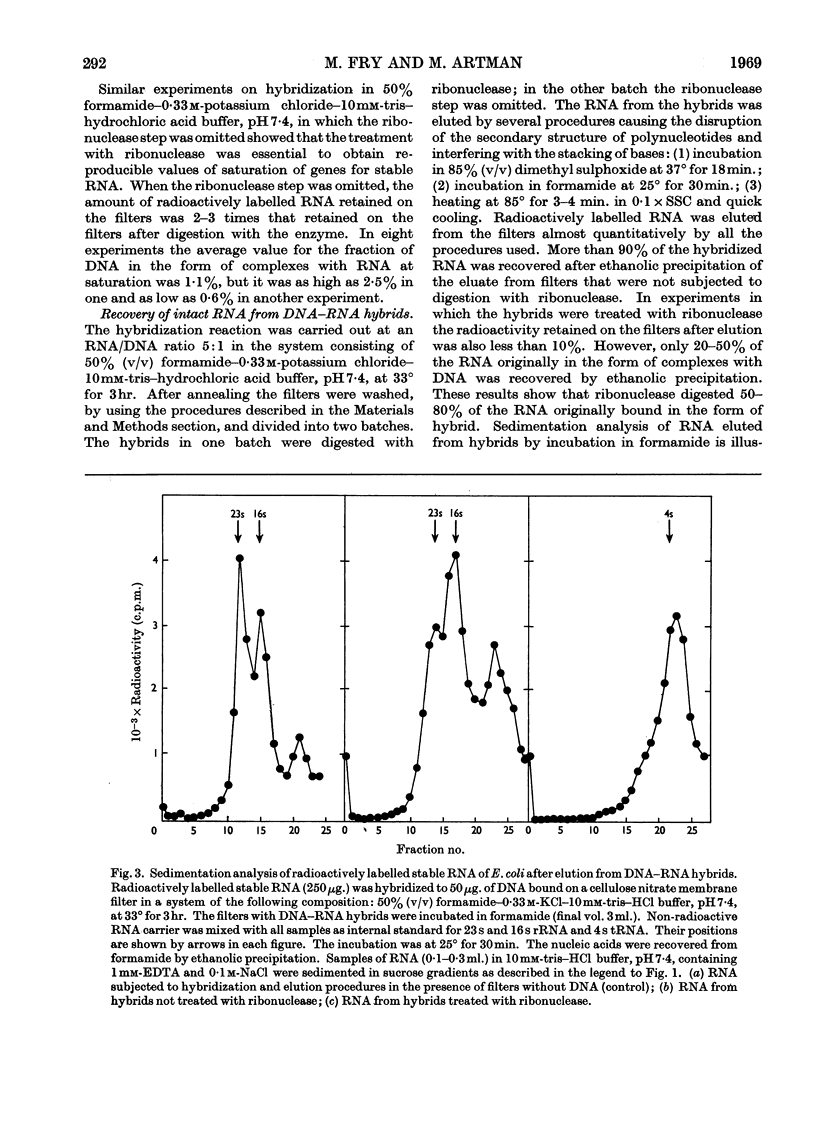
Abstract
A simple and efficient method for hybridization and subsequent recovery of non-fragmented ribosomal RNA from the hybrid is described. The procedure involves annealing of immobilized denatured DNA bound on cellulose nitrate membrane filters to complementary RNA in 50% (v/v) formamide–0·33m-potassium chloride–10mm-tris–hydrochloric acid buffer, pH7·4, at 33° for 3hr. Under these conditions no detectable changes in the sedimentation coefficients of the input RNA were detected. The RNA can subsequently be recovered quantitatively from the hybrid in intact form by incubating the filters in formamide or in 85% (v/v) dimethyl sulphoxide. The applicability of the method for the evaluation of the absolute size of ribosomal RNA cistrons in Escherichia coli DNA and for the determination of the size of messenger RNA molecules is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Huang P. C., Kabat S. Recognition of ribosomal RNA sites in DNA. I. Analysis of the E. coli system. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1490–1498. doi: 10.1073/pnas.53.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Bonner J., Kung G., Bekhor I. A method for the hybridization of nucleic acid molecules at low temperature. Biochemistry. 1967 Dec;6(12):3650–3653. doi: 10.1021/bi00864a005. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M., Artman M. Sedimentation behaviour of rapidly labelled RNA from Escherichia coli. Nature. 1968 Feb 17;217(5129):661–664. doi: 10.1038/217661b0. [DOI] [PubMed] [Google Scholar]
- GIACOMONI D., SPIEGELMAN S. Origin and biologic individuality of the genetic dictionary. Science. 1962 Dec 21;138(3547):1328–1331. doi: 10.1126/science.138.3547.1328. [DOI] [PubMed] [Google Scholar]
- HALL B. D., SPIEGELMAN S. Sequence complementarity of T2-DNA and T2-specific RNA. Proc Natl Acad Sci U S A. 1961 Feb 15;47:137–163. doi: 10.1073/pnas.47.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell P., Smith I., Dubnau D., Marmur J. Isolation and characterization of low molecular weight ribonucleic acid species from Bacillus subtilis. Biochemistry. 1967 Jan;6(1):258–265. doi: 10.1021/bi00853a040. [DOI] [PubMed] [Google Scholar]
- Rodgers A. Magnesium ions and the structure of Escherichia coli ribosomal ribonucleic acid. Biochem J. 1966 Jul;100(1):102–109. doi: 10.1042/bj1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F., Leder P. Messenger RNA: an evaluation. Annu Rev Biochem. 1966;35:195–230. doi: 10.1146/annurev.bi.35.070166.001211. [DOI] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. The identification of the ribosomal RNA cistron by sequence complementarity. II. Saturation of and competitive interaction at the RNA cistron. Proc Natl Acad Sci U S A. 1962 Aug;48:1466–1472. doi: 10.1073/pnas.48.8.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]


