Abstract
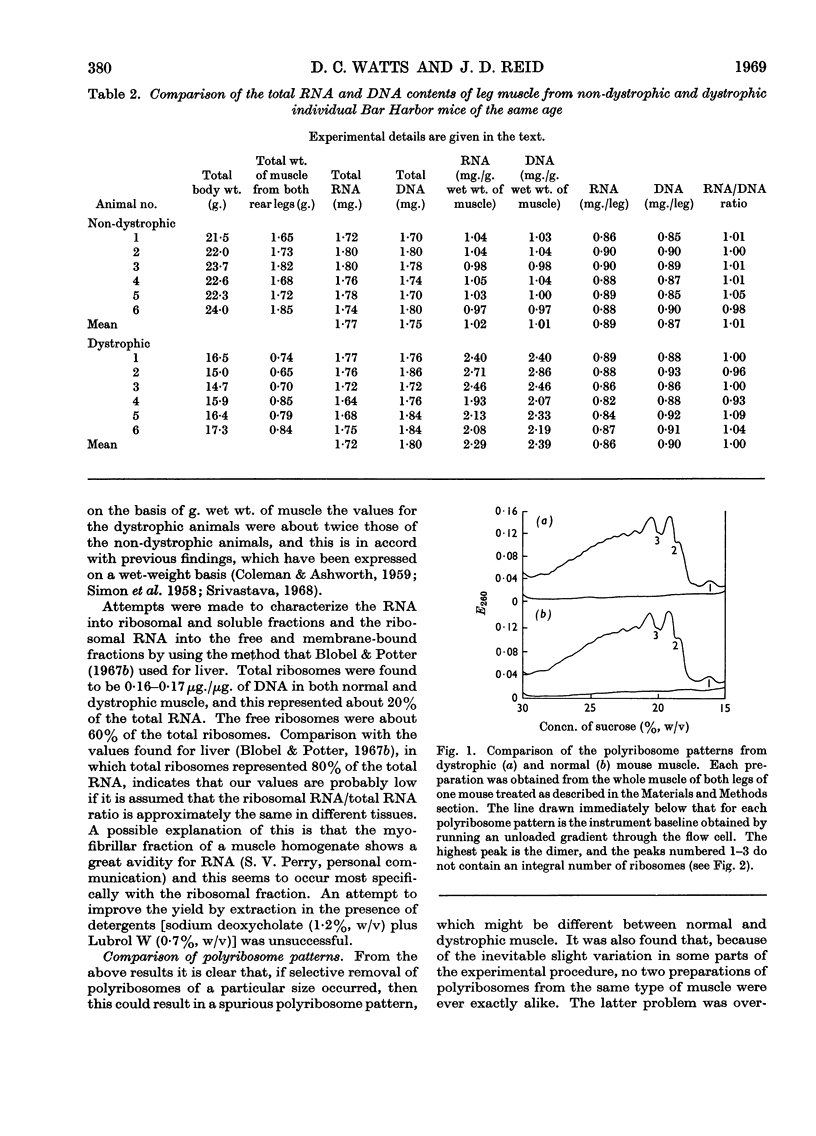
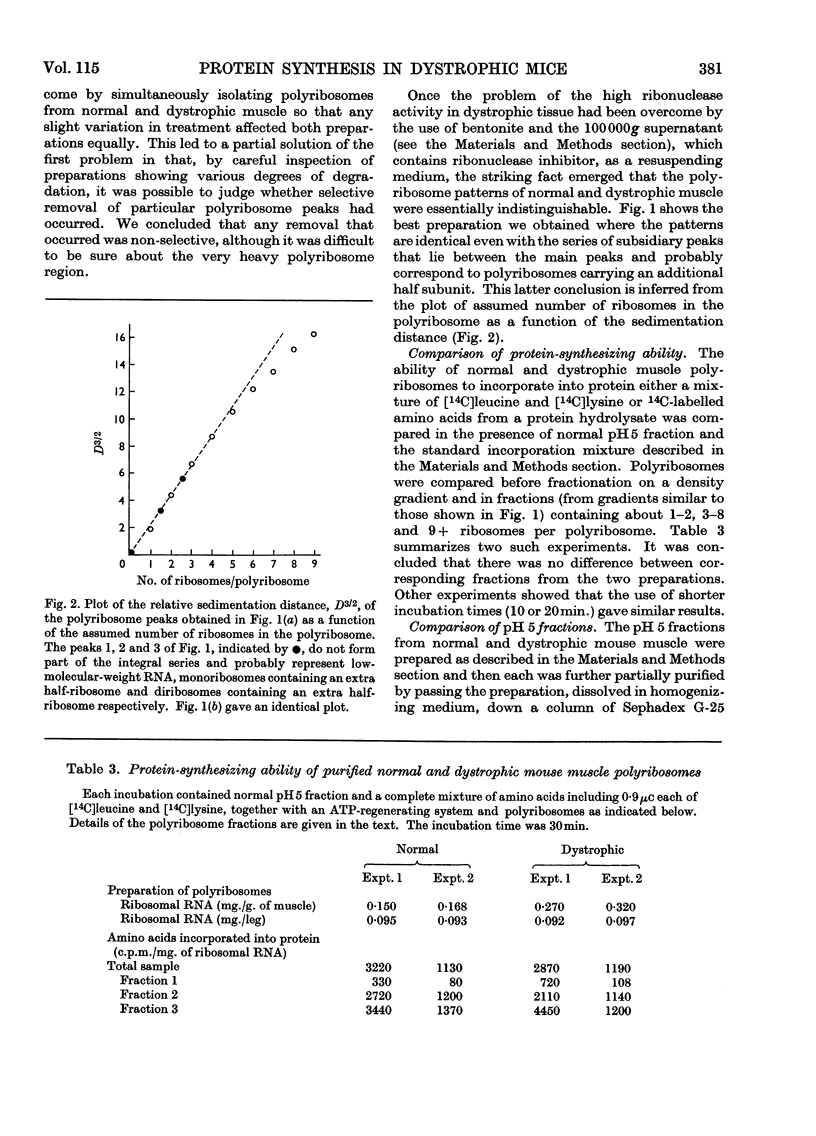
1. Although the total weight of leg muscle increased with the age of a normal mouse the DNA and RNA content per leg did not change significantly. 2. The weight of leg muscle from a dystrophic mouse was only about 45% of that from a normal mouse but the DNA and RNA contents were the same and hence similar DNA/RNA ratios were obtained. 3. The total ribosome contents of normal and dystrophic mice were the same on a whole-leg basis, and for both the free ribosomes were about 60% of the total. However, comparison with similar data from liver suggested that some loss of ribosomes occurred during the isolation procedure. 4. The polyribosome patterns obtained by density-gradient centrifugation were the same for normal and dystrophic muscle, and comparable polyribosome fractions of different sizes obtained from such gradients had similar capacities for the incorporation of radioactive amino acids in a standard protein-synthesizing system. 5. By using a standard protein-synthesizing system with normal polyribosomes similar extents of incorporation were found with normal- or dystrophic-muscle pH5 fraction or partially purified transfer RNA preparation. 6. It is concluded that there is no absolute difference between the protein-synthesizing systems of normal and dystrophic mouse muscle and that the observed apparent differences result from concentration differences caused by changes in muscle volume. 7. A possible cause of the failure of dystrophic muscle to resynthesize myofibrils is also suggested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlinguet L., Srivastava U. Proteolytic enzymes in normal and dystrophic mouse muscle. Can J Biochem. 1966 May;44(5):613–623. doi: 10.1139/o66-074. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Relation of ribonuclease and ribonuclease inhibitor to the isolation of polysomes from rat liver. Proc Natl Acad Sci U S A. 1966 May;55(5):1283–1288. doi: 10.1073/pnas.55.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J Mol Biol. 1967 Jun 14;26(2):279–292. doi: 10.1016/0022-2836(67)90297-5. [DOI] [PubMed] [Google Scholar]
- Breuer C. B., Florini J. R. Amino acid incorporation into protein by cell-free systems from rat skeletal muscle. IV. Effects of animal age, androgens, and anabolic agents on activity of muscle ribosomes. Biochemistry. 1965 Aug;4(8):1544–1550. doi: 10.1021/bi00884a013. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. A microchemical determination of desoxyribonucleic acid. J Biol Chem. 1952 Sep;198(1):297–303. [PubMed] [Google Scholar]
- COLEMAN D. L., ASHWORTH M. E. Incorporation of glycine-I-C14 into nucleic acids and proteins of mice with hereditary muscular dystrophy. Am J Physiol. 1959 Oct;197:839–841. doi: 10.1152/ajplegacy.1959.197.4.839. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- FLORINI J. R. AMINO ACID INCORPORATION INTO PROTEIN BY CELL-FREE PREPARATIONS FROM RAT SKELETAL MUSCLE. I. PROPERTIES OF THE MUSCLE MICROSOMAL SYSTEM. Biochemistry. 1964 Feb;3:209–215. doi: 10.1021/bi00890a012. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Breuer C. B. Amino acid incorporation into protein by cell-free systems from rat skeletal muscle. V. Effects of pituitary growth hormone on activity of ribosomes and ribonucleic acid polymerase in hypophysectomized rats. Biochemistry. 1966 Jun;5(6):1870–1876. doi: 10.1021/bi00870a013. [DOI] [PubMed] [Google Scholar]
- GIRKIN G., FITCH C. D., DINNING J. S. Nucleic acid metabolism in mice with hereditary muscular dystrophy. Arch Biochem Biophys. 1962 Aug;98:224–228. doi: 10.1016/0003-9861(62)90176-5. [DOI] [PubMed] [Google Scholar]
- KECK K. An ultramicro technique for the determination of deoxypentose nucleic acid. Arch Biochem Biophys. 1956 Aug;63(2):446–451. doi: 10.1016/0003-9861(56)90059-5. [DOI] [PubMed] [Google Scholar]
- KRUH J., DREYFUS J. C., SCHAPIRA G., GEY G. O., Jr Abnormalities of muscle protein metabolism in mice with muscular dystrophy. J Clin Invest. 1960 Jul;39:1180–1184. doi: 10.1172/JCI104132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUPNICK A. B., CASA C. M., ROSENKRANTZ H. NUCLEIC ACID CONTENT IN NUTRITIONAL MUSCULAR DYSTROPHY. Arch Biochem Biophys. 1964 Jul 20;106:89–94. doi: 10.1016/0003-9861(64)90160-2. [DOI] [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem. 1954 Jul;209(1):191–201. [PubMed] [Google Scholar]
- MURTHY M. R., RAPPOPORT D. A. BIOCHEMISTRY OF THE DEVELOPING RAT BRAIN. V. CELL-FREE INCORPORATION OF L-(I-14C)LEUCINE INTO MICROSOMAL PROTEIN. Biochim Biophys Acta. 1965 Jan 11;95:121–131. doi: 10.1016/0005-2787(65)90217-0. [DOI] [PubMed] [Google Scholar]
- McCaman M. W. Enzyme studies of skeletal muscle in mice with hereditary muscular dystrophy. Am J Physiol. 1963 Nov;205(5):897–901. doi: 10.1152/ajplegacy.1963.205.5.897. [DOI] [PubMed] [Google Scholar]
- Munro H. N., Fleck A. Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst. 1966 Feb;91(79):78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- NICHOL C. J. CREATINE KINASE IN MUSCLE OF NORMAL AND DYSTROPHIC MICE OF STRAIN 129. Can J Biochem. 1964 Feb;42:243–249. doi: 10.1139/o64-029. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Amino acid-activating enzymes in muscle. Biochem J. 1960 Oct;77:205–208. doi: 10.1042/bj0770205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAPIRA F., DREYFUS J. C. LA CR'EATINE-KINASE DU S'ERUM CHEZ LA SOURIS MYOPATHIQUE. Enzymol Biol Clin (Basel) 1963;79:53–57. [PubMed] [Google Scholar]
- SRIVASTAVA U., BERLINGUET L. ALDOLASE ACTIVITY IN NORMAL AND DYSTROPHIC MOUSE MUSCLE. Can J Biochem. 1964 Sep;42:1301–1305. doi: 10.1139/o64-139. [DOI] [PubMed] [Google Scholar]
- Srivastava U. Biochemical changes in progressive muscular dystrophy. VII. Studies on the biosynthesis of protein and RNA in various cellular fractions of the muscle of normal and dystrophic mice. Can J Biochem. 1968 Jan;46(1):35–41. doi: 10.1139/o68-006. [DOI] [PubMed] [Google Scholar]
- WEST W. T., MURPHY E. D. Histopathology of hereditary, progressive muscular dystrophy in inbred strain 129 mice. Anat Rec. 1960 Jul;137:279–295. doi: 10.1002/ar.1091370306. [DOI] [PubMed] [Google Scholar]
- Watts R. L., Mathias A. P. The use of bentonite in the isolation of plant polyribosomes. Biochim Biophys Acta. 1967;145(3):828–831. doi: 10.1016/0005-2787(67)90142-6. [DOI] [PubMed] [Google Scholar]
- von der DECKEN, CAMPBELL P. N. Amino acid transfer from aminoacyl-ribonucleic acid to serum albumin by the microsome fraction from rat liver. Biochem J. 1962 Mar;82:448–454. doi: 10.1042/bj0820448. [DOI] [PMC free article] [PubMed] [Google Scholar]


