ABSTRACT
Epilepsy, a chronic neurological disorder affecting around 65 million people globally, is characterized by recurrent, unprovoked epileptic seizures. Psilocin, the active metabolite of psilocybin, a well‐known psychedelic compound, has recently gained attention for its potential antidepressant and anxiolytic properties. This study aims to investigate the anticonvulsant effects of psilocin. The study utilizes behavioral seizure models and electrophysiological recordings in mice to assess the anticonvulsant efficacy of psilocin. The pentylenetetrazole (PTZ) test for clonic seizures and the maximal electroshock (MES) test for generalized tonic–clonic seizures are employed. Cortical electrical activity is monitored to provide insights into the compound's effects on neuronal activity. The involvement of kynurenine pathway, opioidergic and nitrergic systems, as well as cannabinoid receptors using agonist/antagonist paradigms. Western blotting was employed to evaluate the expression levels of key receptors and enzymes implicated in psilocin's anticonvulsant effects. The findings indicate a possible modulation of seizure activity by psilocin, with modest doses (3 mg/kg, i.p.) demonstrating potential anticonvulsant effects. Remarkably, the administration of 1‐MT, L‐NAME, naltrexone, sildenafil, and AM‐251 led to a diminishment of the anticonvulsant effects of psilocin, underscoring the involvement of the kynurenine pathway, nitrergic and opioidergic systems, cGMP, and the CB1 receptor in mediating the anticonvulsant effects of psilocin, respectively. Based on western blotting analysis, the upregulation of 5‐HT1A but not 5‐HT2A and the downregulation of IDO and CB1 expression following psilocin administration were observed. Acute administration of psilocin exerts anticonvulsant effects that might be mediated at least in part through the kynurenine pathway, opioidergic, serotonergic, and nitrergic systems.
Keywords: epilepsy, nitric oxide, psilocin, seizures, serotonin
Chemical structure of psilocin and other psychedelic drugs that impact the serotonergic system.

1. Introduction
Epilepsy, a persistent neurological condition, affects over 65 million individuals worldwide. It is typified by frequent, spontaneous epileptic seizures [1]. First‐line treatment typically involves anti‐seizure medications (ASMs); however, around 30%–40% of patients develop refractory epilepsy unresponsive to pharmacologic intervention [2]. Hence, there is a critical need to identify and develop new anticonvulsant agents with novel mechanisms of action [3, 4].
Psilocin (4‐hydroxy‐N, N‐dimethyltryptamine) is a psychedelic compound and the active metabolite of psilocybin [5]. While known for its psychoactive effects [6], some recent evidence suggests psilocin may also have antidepressant‐ and anxiolytic‐like activity properties [7, 8]. However, there is limited research into the anticonvulsant potential of psilocin. Psilocybin primarily activates the various serotonin receptor subtypes, and its metabolite psilocin likely exerts psychedelic effects through the same mechanism [9]. The connection between serotonergic psychedelics and seizures presents a complex picture. It has been noted that high doses of traditional psychedelics like LSD could potentially induce classic psychedelic‐related seizures [10, 11, 12, 13, 14]. Yet, when administered in carefully measured smaller doses, these same substances have shown the capacity to suppress seizures [15, 16]. Unpacking this puzzle carries important clinical implications [13]. Indeed, it opens up avenues for responsible therapeutic applications if the anticonvulsant properties of modest doses can be harnessed. As research into psychedelics resumes following decades of dormancy, resolving this dichotomy, which may imply both pro‐ and anticonvulsant effects depending on the dosage, in their seizure‐related effects offers a promising avenue for investigation. Reconciling their biphasic dose–response profile could uncover novel biological insights [17].
The cannabinoid type 1 (CB1) receptor, a key component of the endocannabinoid system, has garnered significant attention in preclinical studies investigating its role in the modulation of seizures [18]. Accumulating evidence from animal models suggests that the CB1 receptor plays a crucial role in regulating neuronal excitability and seizure susceptibility [1]. Moreover, the opioidergic and nitrergic systems play vital roles in regulating seizure activity, as evidenced by numerous preclinical studies. The opioidergic system, comprising endogenous opioid peptides and their receptors, has been shown to exert both anti‐ and pro‐convulsant effects through various mechanisms [19]. Opioid receptor antagonists, such as naltrexone, have demonstrated modulatory effects on seizure thresholds across multiple animal models [20]. The nitrergic system, which involves nitric oxide (NO) and related signaling pathways, plays a dual role in seizure modulation.
Like opioids, both proconvulsant and anticonvulsant effects of NO have been reported [21], depending on the specific experimental conditions and brain regions involved, highlighting its complex involvement in seizures [22]. Nitric oxide synthase (NOS) enzymes create NO, a gaseous neurotransmitter and messenger molecule [23] that triggers the catalysis of guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP) by soluble guanylate cyclase (sGC). Because sildenafil is a specific inhibitor of phosphodiesterase type 5, it keeps cGMP from degrading, extending, and amplifying the effects of NO [24]. Therefore, we aimed to elucidate the potential interplay between psilocin, NO, and cGMP in modulating seizure activity. Moreover, the kynurenine pathway, an essential component of tryptophan metabolism, has garnered attention for its involvement in neuroinflammation and modulation of excitatory and inhibitory neurotransmission [25]. Given the close relationship between the kynurenine pathway, tryptophan synthesis, and the serotonergic system with psilocin [26], this study aims to investigate the impact of psilocin on the kynurenine pathway.
Here, we used behavioral seizure models and electrophysiological recordings in mice to assess the anticonvulsant effects of psilocin. We evaluated the anticonvulsant properties of various psilocin dosages in clonic seizures generated by pentylenetetrazole (PTZ) [27] and the maximal electroshock (MES)‐induced generalized tonic–clonic seizures as well‐known mouse models of seizure [28]. We also examined the effects of effective anticonvulsant doses on cortical electrical activity [29]. Determining the anticonvulsant efficacy and mechanism of action of psilocin will shed light on this compound as a possible new therapeutic agent for epilepsy.
2. Materials and Methods
2.1. Animals
In the present investigation, 208 adult male Naval Medical Research Institute (NMRI), which are a common outbred strain of laboratory mice used for general‐purpose research, weighing between 23 and 33 g and aged 6 to 8 weeks, were used. There were 26 groups, with eight mice in each group. The Tehran University of Medical Sciences' ethical committee authorized all recommendations that had to do with using animals for any procedure (IR.TUMS.AEC.1402.213). The mice were housed in cages made of polypropylene that had wire mesh coverings and were kept in climate‐controlled environments. This involved preserving a relative humidity of 55% ± 10%, keeping the temperature at 23°C ± 1°C, and following a 12‐h light/dark cycle. Four mice were kept in each cage. The mice were allowed unlimited access to food and tap water during the trial, with the exception of the designated testing intervals.
2.2. Chemicals
The study employed several drugs, psilocin HCl (provided from our medicinal chemist in department of medicinal chemistry, TUMS, Tehran, Iran). Each medicine was administered intraperitoneally (i.p.) after being dissolved in pharmaceutical‐grade normal saline (0.9% NaCl). Pilot projects and earlier investigations [30, 31], pharmacokinetic considerations [5, 32], and injection schedules were used to establish doses, administration strategies, and injection schedules. Every pharmaceutical solution had a fresh preparation on the day of the trial.
2.3. Induction of Seizures
2.3.1. Clonic Seizure Threshold (CST) Assessments Using i.v. PTZ
To measure CST, the PTZ solution (0.5% in saline) was injected into the mice's tail vein using an infusion pump (Harvard, USA) at a consistent rate of 0.5 mL/min. The gauge‐30 needle was kept in the tail vein using tape. Following forelimb clonus, the infusion was stopped without delay. This was followed by whole‐body clonus and loss of equilibrium. The animals were restrained but not anesthetized during the intravenous PTZ infusion procedure. Furthermore, CST has been determined as the lowest dose of PTZ (measured in mg/kg of each mouse) necessary to cause seizure‐like symptoms employing the following formula:
In this paradigm, first we administered psilocin at doses of 0.03, 0.2, 1, 3, 5, 10, and 20 mg/kg to evaluate the dose–response effects on the CST using intravenous PTZ injection. Using 3 mg/kg of psilocin, identified as the most effective dose, we tested the anticonvulsant effect by injecting it 15, 30, and 45 min prior to PTZ intravenous infusion to study the time‐course effect. We investigated the role of the opioidergic system by administering 1 mg/kg of naltrexone 30 min before 3 mg/kg of psilocin [22, 25, 33]. A control group was included that received naltrexone without psilocin. To study the influence of the nitrergic system, we administered aminoguanidine at 100 mg/kg and L‐arginine at 60 mg/kg 30 min prior to the injection of psilocin [25, 34, 35]. We evaluated the effect of the cGMP pathway by administering sildenafil at a dose of 10 mg/kg, 30 min before psilocin injection [36, 37]. Control groups were included, receiving aminoguanidine, L‐arginine, or sildenafil without psilocin. The role of the CB1 receptor was assessed by administering AM‐251 at 1 mg/kg 30 min prior to psilocin injection [18] A control group received AM‐251 without psilocin. Finally, to explore the involvement of the kynurenine pathway, we administered 1‐MT at a dose of 1 mg/kg 30 min before injecting psilocin [38, 39]. A control group received 1‐MT without psilocin.
2.3.2. Seizure Severity Assessment Using i.p. PTZ
Three parameters were evaluated using an intraperitoneal PTZ injection at a dosage of 85 mg/kg, which is now regarded as CD97 for clonic seizures in the literature. The duration of the first seizure delay, the total number of seizures, and the frequency of death within 30 min after a PTZ injection are the first, second, and third, respectively. A psilocin injection was given 30 min before the intraperitoneal (i.p.) injection at dosages of 1, 3, 5, and 10 mg/kg. Of note, similar to the previous model in this paradigm, the psilocin was injected i.p. before the PTZ injection.
2.3.3. Tonic–Clonic Seizures Assessments Using MES
Utilizing an electroconvulsiometer, tonic–clonic seizures were generated in mice by electroshock administered through electrodes attached to the mouse ears at 60 Hz and 50 mA of intermittent current for 1 s. As a measure of seizure activity, the incidence of tonic hindlimb extension (THLE)—180° extension of the mouse hindlimbs with respect to the body axis—was used. Stated differently, the most typical marker of anticonvulsant medication efficacy in MES is THLE inhibition. Psilocin at the doses of 1, 3, 5, and 10 mg/kg was injected 30 min prior to THLE induced by MES.
2.4. Tissue Collection
Following seizure induction using the PTZ i.p. injection paradigm, mice were sacrificed by decapitation. Whole brains were immediately collected and placed on ice to minimize postmortem changes. The brains were rinsed in ice‐cold phosphate‐buffered saline (PBS) to remove residual blood, blotted dry, and snap‐frozen in liquid nitrogen. The samples were stored at −80°C until further analysis. For all experiments, biological replicates were used unless otherwise specified. In the seizure latency and frequency experiments (Figure 4), each n corresponds to an individual animal. For Western blot analysis (Figure 5), each n represents an independent sample derived from a separate animal. Technical replicates were not included in this study.
FIGURE 4.

Effects of psilocin on seizure latency and frequency. (A) Clonic seizure latency in seconds across different doses of psilocin (1, 3, 5, 10 mg/kg). (B) Tonic–clonic seizure latency in seconds across different doses of psilocin (1, 3, 5, 10 mg/kg). The Kruskal–Walli's test followed by Dunn's multiple comparisons test was performed. (C) Frequency of tonic–clonic seizures across different doses of psilocin (1, 3, 5, 10 mg/kg). One‐way ANOVA with post hoc Tukey's multiple comparisons was performed. *p < 0.05, **p < 0.01, p < 0.001, ****p < 0.0001 compared to control. Data are reported as mean ± SEM.
FIGURE 5.

Psilocin's effects on protein expression in brain. Western blot analysis of protein levels in the hippocampus showing effects of psilocin (3 mg/kg) on 5‐HT1A (A), 5‐HT2A (B), CB1 (C), and IDO (D) expression in healthy, PTZ, and psilocin‐treated groups. Bar plots were generated using densitometry analysis of protein bands from n = 3 biological replicates, quantified using ImageJ software. One‐way ANOVA followed by Tukey's post hoc test was performed. *p < 0.05, **p < 0.01, ***p < 0.001, ns, non‐significant.
2.5. Western Blotting for Protein Expression Analysis
The membranes were blocked for 1 h with 5% BSA (Cat No: A‐7888; Sigma Aldrich, MO, USA) in 0.1% Tween 20. They were then incubated with primary antibodies for 1 h at room temperature: Anti‐5‐HT2A receptor (cat no. ab216959, Abcam), Anti‐5‐HT1A receptor (cat no. ab85615, Abcam), Anti‐IDO (cat no. ab311847, Abcam), and Anti‐CB1 receptor (cat no. ab259323, Abcam), all at a concentration of 1:1000. Additionally, membranes were incubated with the anti‐β actin‐loading control antibody (Cat No: ab8227, Abcam). Following three TBST washes, the membranes were exposed to enhanced chemiluminescence (ECL) for 1–2 min after being incubated with the secondary antibody, goat anti‐rabbit IgG H&L (HRP) (cat no. ab6721; Abcam). The levels of protein expression were compared to those of β‐actin. Using Gel Analyzer Version 2010a software (NIH, USA), densitometry analysis of protein bands was carried out. The percentage area under the curve for each band in relation to its matching actin band (normalized to beta‐actin) was calculated, and data were compared across groups as previously stated [40].
2.6. Nitrite Level Using Griess Test
We conducted the nitric oxide (NO) measurement using the Natrix Nitric Oxide (NO) Assay Kit following a standardized protocol. Initially, we prepared our biological samples, including plasma, serum, urine, and tissue homogenates, ensuring all were appropriately processed to remove proteins and debris. For plasma and serum, we centrifuged the samples at 14000 rpm for 10 min, while urine samples were diluted at 1:10 due to high nitrate content. Tissue samples were homogenized and similarly centrifuged. All reagents were equilibrated to room temperature before the assay, and any crystals present were dissolved by gentle warming. We then added 50 μL of each sample or standard to the wells of a 96‐well plate, followed by 50 μL each of Reagent 1 and Reagent 2. The mixture was incubated for 10 min at room temperature to allow the Griess reaction to occur, forming a colored azo compound that correlates with nitrite concentration. After the incubation period, we measured the absorbance of each well at 570 nm using a microplate reader. The absorbance values obtained were used to construct a standard curve, from which we derived the nitrite concentrations of the samples. This curve was based on the provided standard solutions, enabling us to calculate the NO levels in micromolar units.
2.7. Analysis of Electrocorticography (ECoG) in PTZ and Psilocin + PTZ Groups
Electrocorticography (ECoG) was used to capture cerebral cortex activity in animals experiencing seizures in order to assess the impact of psilocin on brain neural activity. Mice were put in a stereotaxic frame and sedated with ketamine and xylazine (100 and 20 mg/kg) prior to electrode insertion. After shaving, the scalp was cleaned. To make the skull visible, a midline incision was made. A‐M Systems Co., US, describes ECoG electrodes as stainless‐steel micro screws attached to a monopolar stainless‐steel electrode wire. The reference and ground electrodes were positioned over the occipital brain, while the recording electrode was implanted over the frontal cortex. Mice were attached to the ECoG recording device (BIODAC ES1721, Trita Health Technology CO., Tehran, Iran) during a two‐week recuperation period. ECoG activity was measured for 15 min prior to the medication injection in order to provide a steady baseline recording. Then, after receiving injections of vehicle + PTZ and psilocin + PTZ, ECoG activity was monitored continuously for 30 min. Analysis of ECoG traces was done to determine how long epileptiform activity lasted. A frequency of less than 1 Hz and an amplitude twice the baseline were required for epileptiform activity Both the length of the whole seizure and the latency to its start were measured. Both the latency to seizure onset and the total seizure duration were measured. To provide a more comprehensive analysis of psilocin's effects on PTZ‐induced seizure activity, the epileptiform discharge duration (s) was also calculated as the sum of the duration of primary and secondary epileptiform discharges. Epileptiform discharges were considered as the fluctuations in the recorded field potentials when their amplitude was twice the baseline and their frequency was higher than 0.25 Hz. To evaluate the impact of psilocybin on PTZ‐induced seizure activity, ECoG data from the PTZ and Psilocin + PTZ groups were compared.
2.8. Statistical Analysis
GraphPad Prism (version 8) and SPSS 16 were utilized for all statistical analyses. Tests for homogeneity of variance were employed to ensure that the distribution of the data was normal. One‐way and two‐way analysis of variance (ANOVA) was performed before using Tukey's post hoc multiple comparison test to assess differences in CST, seizures frequency, and biochemical variables. The data were displayed using mean ± standard error of the mean (S.E.M.), with six mice in each group. However, due to the results of the Shapiro–Wilk test, which indicated that the data were not normally distributed, we employed the non‐parametric Kruskal–Wallis's test, followed by Dunn's post hoc test, to compare the first seizure onset and seizure duration in the i.p. PTZ paradigm and data reported as median with interquartile range. Additionally, the Fisher's exact test was utilized to analyze the data in the MES paradigm with 10 mice in each group. P‐values less than 0.05 were considered statistically significant.
2.9. Nomenclature of Targets and Ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY [41], and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 [42].
3. Results
3.1. Dose–Response and Time‐Course Effects of Psilocin Against PTZ‐Induced Seizures
Figure 1A represents CST across different doses of psilocin (0.03, 0.5, 1, 3, 5, 10, and 20 mg/kg). According to the results of our study, psilocin at the doses of 3 and 5 mg/kg could increase CST, suggesting anticonvulsant activity (F[7, 35] = 6.25, p = 0.0002).
FIGURE 1.
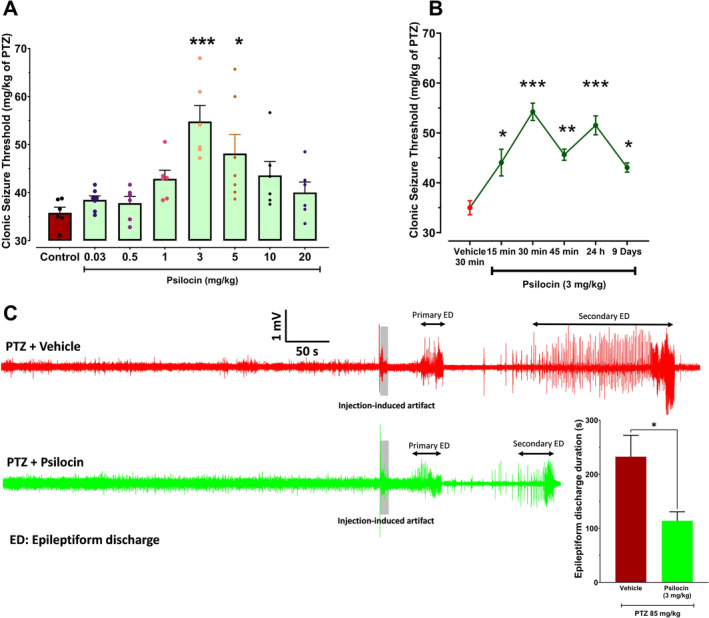
Dose–response and time‐course effects of psilocin on clonic seizure threshold and ECoG analysis. (A) Dose–response effects of psilocin (0.03, 0.5, 1, 3, 5, 10, 20 mg/kg) on clonic seizure threshold measured in mg/kg of PTZ. One‐way ANOVA test followed by Tukey's multiple comparisons test was performed. *p < 0.05, ***p < 0.001 compared to control. (B) Time‐course effects of 3 mg/kg psilocin on CST measured at 15, 30, and 45 min prior to PTZ administration. *p < 0.05, ***p < 0.001 compared to vehicle at 30 min. (C) Representative ECoG traces from PTZ (top, red) and Psilocin + PTZ (bottom, green) groups. The gray shaded areas indicate the time of PTZ injection and the injection‐induced artifact. In the PTZ + Psilocin group a reduction in the epileptiform discharge duration was observed compared to PTZ group, suggesting a protective effect of psilocin against PTZ‐induced seizures. The bar diagram on the below right showed the quantitative group data in all animals (n = 4 in PTZ and n = 6 in PTZ + Psilocin group; *p < 0.05).
Figure 2B displays the time course investigation of psilocin's anti‐seizure action at the dose of 3 mg/kg, which was the most effective dosage (F[5, 27] = 15.61, p < 0.0001). CST was unaffected by psilocin (3 mg/kg) administered 15 min before PTZ infusion (p > 0.05). The CST was significantly impacted by the same amount of psilocin administered 30 min before seizure induction (***p < 0.0001), whereas the effect was less pronounced but still significant 45 min prior to the test (*p < 0.01). Based on the obtained data, there is a significant (p < 0.0001) elevation in CST 24 h following acute injection of psilocin (3 mg/kg). Moreover, 9 days after a single i.p. injection of psilocin at the dose of 3 mg/kg, we still observed a significant anticonvulsant effect (p < 0.05).
FIGURE 2.

Effect of pharmacological pretreatments on psilocin's anticonvulsant activity. (A) AM‐251 significantly diminished the anticonvulsant effects of psilocin, **p < 0.01, (B) 1‐MT pretreatment significantly reduced the anticonvulsant effects of psilocin, *p < 0.01, (C) Sildenafil pretreatment significantly alter the anticonvulsant effects of psilocin. *p < 0.01, (D) Naltrexone pretreatment significantly attenuated the anticonvulsant effects of psilocin, *p < 0.01. Following the Two‐way ANOVA test, the Tukey multiple comparisons test was run.
In Figure 1C, the ECoG activity following the administration of PTZ shows the characteristic spike‐and‐wave discharges associated with seizure activity. ECoG recordings revealed distinct differences in seizure activity between the PTZ and Psilocin + PTZ groups. In the PTZ group (top trace, red), epileptiform discharges began shortly after injection, characterized by high‐amplitude, high‐frequency waveforms. The duration of epileptiform activity was prolonged, indicating sustained seizure events. Conversely, in the Psilocin + PTZ group (bottom trace, green), epileptiform activity was delayed, with reduced amplitude and frequency, and the overall duration of discharges was shorter compared to the PTZ group (p < 0.014). These findings suggest that psilocin attenuates PTZ‐induced seizure activity by reducing both the intensity and duration of epileptiform discharges.
While there appeared to be differences in follow‐up timing between groups, both were monitored under identical conditions, with injection timing being the only variable. This ensured that observed differences in ECoG parameters were attributable solely to treatment effects.
3.2. Mechanisms Underlying the Anticonvulsant Effects of Psilocin
Figure 2A shows that co‐administration of the CB1 receptor antagonist AM‐251 significantly reduced the clonic seizure threshold in the psilocin‐treated group (F[1, 16] = 11.91, p = 0.0033) compared to the vehicle‐treated group (p < 0.01). This suggests that blocking CB1 receptors diminishes the anticonvulsant effect of psilocin.
Figure 2B demonstrates that the IDO inhibitor 1‐MT also significantly lowered the clonic seizure threshold in the psilocin‐treated group (F[1, 16] = 12.57, p = 0.0027) compared to the vehicle group (p < 0.05), indicating that IDO activity might contribute to the anticonvulsant properties of psilocin.
Figure 2C illustrates that the phosphodiesterase‐5 inhibitor sildenafil significantly decreased the clonic seizure threshold in the psilocin‐treated group (F[1, 16] = 11.29, p = 0.0040) compared to the vehicle group (p < 0.05), suggesting that increasing cyclic GMP levels interferes with psilocin's anticonvulsant effects.
Figure 2D shows that the opioid receptor antagonist naltrexone significantly reduced the clonic seizure threshold in the psilocin‐treated group (F[1, 16] = 8.162, p = 0.0114) compared to the vehicle group (p < 0.01), highlighting the potential involvement of opioid receptors in the mechanism of action of psilocin.
3.3. Involvement of Nitrergic System on the Anticonvulsant Effects of Psilocin
Figure 3A shows that co‐administration of the AG, an nNOS inhibitor, did not reduce the clonic seizure threshold in the psilocin‐treated group (F[1, 16] = 7.525, p = 0.0144) compared to the vehicle‐treated group (p > 0.05).
FIGURE 3.

Role of nitrergic system in psilocin's anticonvulsant effects. (A) Pretreatment effects of aminoguanidine (AG, 1 mg/kg, i.p.) or its vehicle (saline) on the anticonvulsant effects of psilocin (3 mg/kg, i.p.) on CST. Aminoguanidine/psilocin versus saline/psilocin were compared with each other p > 0.05 (Two‐way ANOVA). (B) Effects of an ineffective dosage of L‐arginine (60 mg/kg, i.p.) on effective dose of psilocin (3 mg/kg, i.p.) on CST. When comparing the saline/psilocin and L‐arginine/psilocin groups, ## p < 0.001 was found (Two‐way ANOVA). (C) Nitrite level in healthy, PTZ administrated group, and psilocin group *p < 0.05 and **p < 0.01. Data are reported as mean ± SEM and each group contains six mice.
Figure 3B demonstrates that L‐arginine significantly lowered the clonic seizure threshold in the psilocin‐treated group (F[1, 15] = 7.245, p = 0.0167) compared to the vehicle group (p < 0.01), indicating that the nitrergic system might contribute to the anticonvulsant properties of psilocin (p < 0.01).
Figure 3C indicates the level of nitrite in the brain of mice. According to data following i.p. administration of PTZ, there is an increase in nitrite levels in the brain (p < 0.01). However, psilocin (3 mg/kg) exerts protective effects and was able to reduce the elevated level of nitrite significantly (F[2, 6] = 34.66, p = 0.0005, and p < 0.01 when compared to PTZ control group).
3.4. Effects of Psilocin on Seizures Induced by i.p. Injection of PTZ
The latency for the start of the first clonic seizure and the incidence of tonic–clonic seizures were dramatically increased (Figure 4A,B) at dosages of 3 and 5 mg/kg of psilocin, but the frequency of clonic seizures throughout the 1800s was decreased (Figure 4C).
Nevertheless, there was no discernible variation in mortality among the various psilocin‐treated groups (Table 1).
TABLE 1.
Mortality rate comparison between groups in i.p. PTZ paradigm following various doses of psilocin administration.
| Groups | Death (%) | p |
|---|---|---|
| Vehicle | 33.34% | — |
| Psilocin 1 mg/kg | 33.34% | 0.9999 |
| Psilocin 3 mg/kg | 0% | 0.0606 |
| Psilocin 5 mg/kg | 33.34% | 0.9999 |
| Psilocin 10 mg/kg | 50% | 0.9999 |
| Psilocin 3 mg/kg (24‐h interval) | 33.34% | 0.9999 |
| Psilocin 3 mg/kg (1‐week interval) | 16.67% | 0.2424 |
Note: Groups were compared to the control group using Fisher's exact test. n = 6.
3.5. Molecular Assessments Regarding Protein Expression Following i.p. Injection of PTZ
Western blot analysis revealed that psilocin treatment (3 mg/kg) resulted in significant changes in the expression of several proteins in the whole brain of mice (Figure 5). Psilocin significantly increased the levels of 5‐HT1A (p < 0.0086) but not 5‐HT2A (p < 0.33) receptors compared to the PTZ group (**p < 0.01, p > 0.05, respectively).
The expression of CB1 receptors (p < 0.005) was also significantly elevated in the psilocin‐treated group compared to PTZ controls (*p < 0.01).
Additionally, IDO expression (p < 0.002) was significantly reduced in the psilocin‐treated group compared to PTZ controls (**p < 0.01), suggesting a modulatory effect on the kynurenine pathway.
3.6. Effects of Psilocin on Tonic–Clonic Seizures Induced by MES
In the MES‐induced tonic–clonic seizure model, psilocin (1, 3, and 10 mg/kg) treatment could protect the mice against the development of tonic seizure, as indicated in Table 2. Psilocin at these doses considerably prevents the mice from mortality. It should be mentioned that each group consisted of 10 mice.
TABLE 2.
Effects of psilocin on seizure incidence as well as mortality following MES‐induced tonic–clonic seizures in mice.
| Groups | Seizure incidence (%) | p | Death (%) | p |
|---|---|---|---|---|
| Vehicle | 90% | — | 70% | — |
| Psilocin 0.5 mg/kg | 40% | 0.0573 | 30% | 0.1789 |
| Psilocin 1 mg/kg | 20% | 0.0055 | 20% | 0.0698 |
| Psilocin 3 mg/kg | 10% | 0.0011 | 0% | 0.0031 |
| Psilocin 5 mg/kg | 30% | 0.0198 | 0% | 0.0031 |
| Psilocin 10 mg/kg | 50% | 0.1409 | 30% | 0.1789 |
| Psilocin 3 mg/kg (24‐h interval) | 30% | 0.0198 | 10% | 0.0198 |
| Psilocin 3 mg/kg (1‐week interval) | 60% | 0.3034 | 10% | 0.0198 |
Note: Groups were compared to the control group using Fisher's exact test. n = 10.
Moreover, similar to the first model, the mechanism underlying the anticonvulsant effects of psilocin in this paradigm was also investigated (Table 3).
TABLE 3.
Mechanism underlying psilocin anticonvulsant effects on MES‐induced tonic–clonic seizures in mice regarding seizure incidence as well as mortality following.
| Pretreatment | Groups | Seizure incidence (%) | p | Death (%) | p |
|---|---|---|---|---|---|
| — | Vehicle | 90% | — | 70% | — |
| — | Psilocin 3 mg/kg | 10%* | 0.0011 | 0%* | 0.0031 |
| L‐arginine 60 mg/kg | Vehicle | 70% | 0.5820 | 60% | 0.9999 |
| Psilocin 3 mg/kg | 70%** | 0.0198 | 50%** | 0.0325 | |
| AG 100 mg/kg | Vehicle | 50% | 0.1409 | 40% | 0.3698 |
| Psilocin 3 mg/kg | 50% | 0.1409 | 30% | 0.2105 | |
| Naltrexone 1 mg/kg | Vehicle | 60% | 0.3034 | 50% | 0.6499 |
| Psilocin 3 mg/kg | 50% | 0.1409 | 60%** | 0.0108 | |
| Sildenafil 10 mg/kg | Vehicle | 80% | 0.99 | 70% | 0.9999 |
| Psilocin 3 mg/kg | 60% | 0.0573 | 50%** | 0.0325 | |
| 1‐MT (1 mg/kg) | Vehicle | 70% | 0.5820 | 70% | 0.9999 |
| Psilocin 3 mg/kg | 80%*** | 0.0055 | 70%*** | 0.0031 | |
| AM‐251 (1 mg/kg) | Vehicle | 60% | 0.3034 | 40% | 0.3698 |
| Psilocin 3 mg/kg | 90%*** | 0.0011 | 80%**** | 0.0007 |
Note: Combination therapy groups with psilocin 3 mg/kg were compared to the psilocin 3 mg/kg and others compared with control group (vehicle only) using Fisher's exact test. *p < 0.01 when compared to vehicle control group. Also, **p < 0.05, ***p < 0.01, and ****p < 0.001 when compared to psilocin 3 mg/kg alone group. n = 10.
4. Discussion
In this study, we showed that acute administration of psilocin has an anticonvulsant effect against PTZ‐induced clonic seizures and MES‐induced tonic–clonic seizures in mice. According to our results, psilocin (3, 5 mg/kg. i.p.) increased CST considerably, whereas higher (10 and 20 mg/kg) and lower (0.03, 0.5, and 1 mg/kg) dosages had no significant effect following i.v. infusion of PTZ. The same dosages of psilocin exert anticonvulsant effects against i.p. injection of PTZ. Our findings revealed that psilocin (3 and 5 mg/kg) exerts anticonvulsant effects against MES‐induced generalized tonic–clonic seizures and inhibits THLE. To the best of our knowledge, this is the first study investigating the clonic seizure threshold following psilocin administration in an animal model. The administration of PTZ is a well‐documented method for inducing clonic seizures in rodents, which mainly act by increasing the activity of epileptogenic areas of the forebrain. Both PTZ and MES models are valuable methods for screening newly discovered ASMs. Although based on the previous investigations, the PTZ model is believed to be more accurate in the prediction of human exposure [43]. Changes in the pattern of discharge, such as fewer spike‐and‐wave complexes, would suggest an impact on seizure dynamics. Change in seizure patterns in the psilocin + PTZ trace compared to the PTZ trace alone suggests an anticonvulsant effect of psilocin.
It is widely established that psilocybin induces robust and long‐lasting improvements in depressive symptoms when administered in a controlled setting. A single dose of psilocybin has been shown to alleviate treatment‐resistant depression for up to 6 months in a substantial proportion of patients [44, 45, 46]. The underlying mechanisms behind psilocybin's enduring antidepressant effects are not fully elucidated, but may involve promoting neuroplasticity, neurogenesis, and neural network re‐organization via BDNF [47, 48]. In our study, we found that not only were anticonvulsant effects present 24 h after a single systemic injection of psilocin in mice, but these effects persisted for at least 9 days post‐administration. This suggests that the therapeutic utility of these compounds may extend beyond their well‐known antidepressant actions, potentially offering valuable new treatment options for neurological disorders characterized by seizures or hyperexcitability, such as epilepsy. The persistent nature of the anticonvulsant effects is an intriguing area for further research, as elucidating the underlying neurobiological mechanisms could open up new avenues for the development of innovative therapies.
Of note, a recent study reported that using psychedelic mushrooms resulted in exacerbation of epilepsy in a patient with a history of focal seizures. Although these exacerbations took place only after consumption of large amounts of magic mushrooms, lower dosages did not change the baseline seizure frequency significantly [11]. Therefore, as mentioned earlier, in this study, we examined low doses to evaluate the beneficial effects. Supporting the above‐mentioned results, another study demonstrated that psychedelics could possibly be associated with a higher rate of epilepsy exacerbations, particularly in people with a prior history of epilepsy [13]. Anxiolytic effects of psilocin have been demonstrated by both clinical and preclinical studies reporting that these drugs have more efficacy compared with common antidepressants such as SSRIs without producing neurotoxicity [7, 49].
Current clinical and experimental studies highlight the importance of the serotonergic pathway in the treatment of epilepsy, as 5‐HT receptor subtypes are involved in different types of epilepsy, and current treatments achieve their therapeutic effect through targeting distinct 5‐HT receptors [50]. Although several studies revealed the significant reduction of the 5‐HT1A receptors in patients with temporal lobe epilepsy, it is believed that these changes might be due to the comorbid depression in these patients [51]. In line with our results, another study revealed the crucial role of 5‐HT1A receptors in the anticonvulsant effects of 8‐OH‐DPAT and Indorenate in rats [52]. Also, earlier evidence suggests that 5‐HT2A receptors don't appear to be involved in epilepsy pathogenesis [53]. Moreover, it is believed that the same receptors are involved in the psychedelic effects of psilocin [54]. Our results revealed the modulatory effect of psilocin on 5‐HT1A receptors and Based on the obtained data, there was no significant alteration in 5‐HT2A expression following acute psilocin administration. The psychedelic effects of psilocin are mediated through the activation of 5‐HT2A receptors, which aligns with existing literature [55]. However, our study's data indicate that the anticonvulsant effects of psilocin are not mediated through this receptor subtype. This suggests a dissociation between the psychoactive and therapeutic mechanisms of psilocin. The dissociation between the psychoactive and anticonvulsant effects of psilocin is a notable observation. It implies that the therapeutic benefits of low doses of psilocin in epilepsy treatment can potentially be harnessed without inducing the psychedelic experiences typically associated with serotonergic psychedelics. This distinction could be significant for patients who may benefit from the anticonvulsant properties of psilocin (at low doses) without experiencing unwanted psychoactive effects [56].
CB1 has been demonstrated to exert an anticonvulsant effect in both clinical and preclinical studies [57], and the administration of arachidonyl‐2′‐chloroethylamide (ACEA) as an agonist of the CB1 receptor enhanced the anticonvulsant activity of valproate against electroshock [58]. A study by Lopes et al. reported the contribution of the endocannabinoid system and the expression of CB1 receptors in acute and chronic seizures of the amygdala and hippocampus [59]. Based on the previous evidence of the crucial role of cannabinoid receptors in the anticonvulsant effect of several drugs, we hypothesized that these receptors may contribute to the anticonvulsant effect of psilocin. Following the administration of AM‐251, which is an inverse agonist of CB1 receptors, we observed a significant reduction in the anticonvulsant properties of psilocin, suggesting that psilocin may exert its anticonvulsant effects partially through the activation of CB1 receptors. In line with these results, according to our data, the expression of CB1 receptors was considerably lower in psilocin compared to the PTZ group. Further research is needed to elucidate the precise mechanisms underlying these interactions and their implications for epilepsy treatment. We hypothesized that the anticonvulsant properties of psilocin may be associated with IDO activity, as it is one of the most important enzymes that shifts the metabolism of tryptophan to serotonin in the kynurenine pathway. A previous clinical article demonstrated that increased activity of IDO is associated with different types of epilepsy, such as idiopathic generalized epilepsy and juvenile myoclonic epilepsy [60]. In accordance with these findings, another study found that the deletion of the IDO gene suppressed seizures and decreased neurotoxic metabolites significantly [61]. Moreover, there is preclinical evidence regarding the association between the IDO enzyme and depressive‐like behavior previously observed in rats with temporal‐lobe epilepsy [62]. In our study, pretreatment with 1‐MT, the inhibitor of IDO, reduced the anticonvulsant effect of psilocin.
Nitric oxide plays a crucial role as a neuromodulator and neurotransmitter in the mammalian central nervous system and is proved to be involved in epileptogenesis [63, 64]. Moreover, this molecule takes part in neuroinflammation, which leads to neuronal toxicity and death [65, 66]. According to the results as mentioned above, we aimed to investigate the nitric oxide (NO)/cyclic guanylate monophosphate cGMP pathway as a possible contributor to the anticonvulsant properties of psilocin. Our findings show that the administration of sildenafil as a cGMP inducer decreased the anticonvulsant effects of psilocin significantly. Opioid receptors are known to modulate pain, mood, and neuronal excitability, and their interaction with serotonergic systems can influence seizure susceptibility. Moreover, previous studies revealed the modulatory effect of the opioidergic system on seizure activity and the upregulation of opioid receptors following spontaneous seizures [67]. In our study, naltrexone significantly reduced the anticonvulsant effects of psilocin, highlighting the potential involvement of opioid receptors in the mechanism of action of psilocin.
Despite the promising findings regarding psilocin's anticonvulsant effects, several limitations should be acknowledged. First, although we successfully tested the involvement of 5‐HT1A receptors, we were unable to obtain the selective 5‐HT2A antagonist, ketanserin, due to logistical constraints and financial limitations, compounded by embargoes on importing certain research materials. This restricted our ability to fully evaluate the role of 5‐HT2A receptors in psilocin's anticonvulsant mechanisms, though existing literature suggests their primary role in mediating psychoactive rather than anticonvulsant effects.
Second, although we selected doses of receptor antagonists based on well‐established pharmacological studies, we acknowledge that the possibility of off‐target effects, particularly at higher doses, cannot be entirely excluded. We mitigated this concern by selecting doses known for their specificity in blocking the targeted receptors. However, further studies with receptor binding assays could more precisely determine receptor occupancy to confirm the exclusivity of each antagonist's effect.
Additionally, this study utilized only male mice, which may limit the generalizability of the findings to female populations or to human clinical trials. Sex‐based differences in seizure susceptibility and drug metabolism are well documented, and future studies should include both male and female subjects to ensure a more comprehensive understanding of psilocin's effects.
Lastly, while our findings provide strong evidence for the anticonvulsant properties of psilocin in acute models of seizures, the long‐term effects of chronic administration and its potential for tolerance or dependence were not explored. Further investigation into these aspects, as well as clinical trials, will be essential to validate psilocin's therapeutic potential for epilepsy in human populations.
To our knowledge, this is the first study investigating the anticonvulsant properties of psilocin; however, it encounters some drawbacks. One of the limitations of our research is the exclusive use of male mice in our experiments, so using female mice in a larger sample size could impact our conclusions. Additionally, considering that this was an animal study, our results might not be fully applicable to clinical trials, which are necessary to validate the therapeutic potential of psilocin for epileptic patients.
5. Conclusion
The results of the present study revealed the anticonvulsant properties of psilocin in doses of 3 and 5 mg/kg in PTZ and MES seizure models in mice. We demonstrated that psilocin exerts its effects, possibly via modulating 5‐HT1A and CB1 receptors, the NO/cGMP pathway, and the IDO enzyme. Our findings indicate that psilocin exhibits significant therapeutic potential in the treatment of epilepsy; however, these results should be treated cautiously and need to be validated by further studies.
Author Contributions
M.B. performed the animal study, wrote some part of article. M.A.M. supervised animal study, wrote the article, visualized and analyzed the data. A.L. consulted methods. R.M.J. consulted methods, edited the essay critically. H.S. consulted methods and MES Seizure. N.H. performed the ECoG. J.M‐Z. consulted methods the ECoG. A.A. edited the essay critically. A.F. was responsible for resources and synthesis. A.R.D. is the correspondence and principal investigator.
Ethics Statement
All experimental steps were performed according to the authorized instructions of animal care and ethics of Tehran University of Medical Sciences (IR.TUMS.AEC.1402.213). We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Data S1. Supporting Information.
Funding: This work was supported by a grant from the Experimental Medicine Research Center, Tehran University of Medical Sciences, by the Iran National Science Foundation (INSF) with grant no. (1402‐4‐209‐69237).
Mohammad Balabandian and Mohammad Amin Manavi contributed equally as first authors.
Contributor Information
Mohammad Amin Manavi, Email: ma-manavi@alumnus.tums.ac.ir.
Razieh Mohammad Jafari, Email: rmjafari@sina.tums.ac.ir.
Ahmad Reza Dehpour, Email: dehpour@yahoo.com, Email: dehpoura@sina.tums.ac.ir.
Data Availability Statement
The data of the present study are available from the corresponding author on reasonable request.
References
- 1. GBD 2016 Epilepsy Collaborators , “Global, Regional, and National Burden of Epilepsy, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016,” Lancet Neurology 18, no. 4 (2019): 357–375, 10.1016/s1474-4422(18)30454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mamo B., Feyissa A. M., Mengesha T., Ayele B. A., and Mamushet Yifru Y., “Association Between Cognitive Impairment and Antiseizure Medication Adherence Among People With Epilepsy in Addis Ababa, Ethiopia,” Epilepsy & Behavior 152 (2024): 109651, 10.1016/j.yebeh.2024.109651. [DOI] [PubMed] [Google Scholar]
- 3. Mendoza‐Madrigal R., González‐Trujano M. E., Onofre‐Campos D., Moreno‐Pérez G. F., Castellanos‐Mijangos J. G., and Martínez‐Vargas D., “Electroencephalographic Profile of Salvia Amarissima Ortega and Amarisolide A in the Absence and Presence of PTZ‐Induced Seizures in Mice,” Biomedicine & Pharmacotherapy 173 (2024): 116352, 10.1016/j.biopha.2024.116352. [DOI] [PubMed] [Google Scholar]
- 4. Manavi M. A., Mohammad Jafari R., Shafaroodi H., Sharifzadeh M., and Dehpour A. R., “Ivermectin as a Promising Therapeutic Option for Onchocerciasis‐Associated Epilepsy,” Epilepsia Open 10 (2024): 361–367, 10.1002/epi4.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones N. T., Wagner L., Hahn M. C. P., Scarlett C. O., and Wenthur C. J., “In Vivo Validation of Psilacetin as a Prodrug Yielding Modestly Lower Peripheral Psilocin Exposure Than Psilocybin,” Frontiers in Psychiatry 14 (2023): 1303365, 10.3389/fpsyt.2023.1303365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dodd S., Norman T. R., Eyre H. A., et al., “Psilocybin in Neuropsychiatry: A Review of Its Pharmacology, Safety, and Efficacy,” CNS Spectrums 28, no. 4 (2022): 1–11, 10.1017/s1092852922000888. [DOI] [PubMed] [Google Scholar]
- 7. Hernandez‐Leon A., Escamilla‐Orozco R. I., Tabal‐Robles A. R., et al., “Antidepressant‐ and Anxiolytic‐Like Activities and Acute Toxicity Evaluation of the Psilocybe Cubensis Mushroom in Experimental Models in Mice,” Journal of Ethnopharmacology 320 (2024): 117415, 10.1016/j.jep.2023.117415. [DOI] [PubMed] [Google Scholar]
- 8. Takaba R., Ibi D., Yoshida K., et al., “Ethopharmacological Evaluation of Antidepressant‐Like Effect of Serotonergic Psychedelics in C57BL/6J Male Mice,” Naunyn‐Schmiedeberg's Archives of Pharmacology 397, no. 5 (2023): 3019–3035, 10.1007/s00210-023-02778-x. [DOI] [PubMed] [Google Scholar]
- 9. Sherwood A. M., Burkhartzmeyer E. K., Williamson S. E., Baumann M. H., and Glatfelter G. C., “Psychedelic‐Like Activity of Norpsilocin Analogues,” ACS Chemical Neuroscience 15, no. 2 (2024): 315–327, 10.1021/acschemneuro.3c00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miliano C., Marti M., Pintori N., et al., “Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I‐NBOMe,” Frontiers in Pharmacology 10 (2019): 1406, 10.3389/fphar.2019.01406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blond B. N. and Schindler E. A. D., “Case Report: Psychedelic‐Induced Seizures Captured by Intracranial Electrocorticography,” Frontiers in Neurology 14 (2023): 1214969, 10.3389/fneur.2023.1214969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nayak S. M., Gukasyan N., Barrett F. S., Erowid E., Erowid F., and Griffiths R. R., “Classic Psychedelic Coadministration With Lithium, but Not Lamotrigine, Is Associated With Seizures: An Analysis of Online Psychedelic Experience Reports,” Pharmacopsychiatry 54, no. 5 (2021): 240–245, 10.1055/a-1524-2794. [DOI] [PubMed] [Google Scholar]
- 13. Simonsson O., Goldberg S. B., Chambers R., Osika W., Long D. M., and Hendricks P. S., “Prevalence and Associations of Classic Psychedelic‐Related Seizures in a Population‐Based Sample,” Drug and Alcohol Dependence 239 (2022): 109586, 10.1016/j.drugalcdep.2022.109586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malcolm B. and Thomas K., “Serotonin Toxicity of Serotonergic Psychedelics,” Psychopharmacology 239, no. 6 (2022): 1881–1891, 10.1007/s00213-021-05876-x. [DOI] [PubMed] [Google Scholar]
- 15. Meldrum B. S. and Naquet R., “Effects of Psilocybin, Dimethyltryptamine and Various Lysergic Acid Derivatives on Photically‐Induced Epilepsy in the Baboon (Papio papio),” British Journal of Pharmacology 40, no. 1 (1970): 144–145. [PMC free article] [PubMed] [Google Scholar]
- 16. Cowan A. and Watson T., “Lysergic Acid Diethylamide Antagonizes Shaking Induced in Rats by Five Chemically Different Compounds,” Psychopharmacology 57, no. 1 (1978): 43–46, 10.1007/BF00426956. [DOI] [PubMed] [Google Scholar]
- 17. Javadian N., Rahimi N., Javadi‐Paydar M., Doustimotlagh A. H., and Dehpour A. R., “The Modulatory Effect of Nitric Oxide in Pro‐ and Anti‐Convulsive Effects of Vasopressin in PTZ‐Induced Seizures Threshold in Mice,” Epilepsy Research 126 (2016): 134–140, 10.1016/j.eplepsyres.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 18. Haj‐Mirzaian A., Ramezanzadeh K., Afshari K., et al., “Activation of ATP‐Sensitive K‐Channel Promotes the Anticonvulsant Properties of Cannabinoid Receptor Agonist Through Mitochondrial ATP Level Reduction,” Epilepsy & Behavior 93 (2019): 1–6, 10.1016/j.yebeh.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 19. Amini‐Khoei H., Rahimi‐Balaei M., Amiri S., et al., “Morphine Modulates the Effects of Histamine H1 and H3 Receptors on Seizure Susceptibility in Pentylenetetrazole‐Induced Seizure Model of Mice,” European Journal of Pharmacology 769 (2015): 43–47, 10.1016/j.ejphar.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 20. Zamanian G., Shayan M., Rahimi N., et al., “Interaction of Morphine Tolerance With Pentylenetetrazole‐Induced Seizure Threshold in Mice: The Role of NMDA‐Receptor/NO Pathway,” Epilepsy & Behavior 112 (2020): 107343, 10.1016/j.yebeh.2020.107343. [DOI] [PubMed] [Google Scholar]
- 21. Homayoun H., Khavandgar S., Namiranian K., Gaskari S. A., and Dehpour A. R., “The Role of Nitric Oxide in Anticonvulsant and Proconvulsant Effects of Morphine in Mice,” Epilepsy Research 48, no. 1–2 (2002): 33–41, 10.1016/s0920-1211(01)00316-3. [DOI] [PubMed] [Google Scholar]
- 22. Jourian S., Rahimi M., Manavi M. A., et al., “Possible Interaction of Opioidergic and Nitrergic Pathways in the Anticonvulsant Effect of Ivermectin on Pentylenetetrazole‐Induced Clonic Seizures in Mice,” Neurochemical Research 48, no. 3 (2023): 885–894, 10.1007/s11064-022-03804-9. [DOI] [PubMed] [Google Scholar]
- 23. Goudarzi S., Mohammad Jafari R., Farsiu N., et al., “Protective Effects of Licofelone on Scopolamine‐Induced Spatial Learning and Memory Impairment by Enhancing Parkin‐Dependent Mitophagy and Promotion of Neural Regeneration and in Adult Mice,” European Journal of Pharmacology 984 (2024): 177025, 10.1016/j.ejphar.2024.177025. [DOI] [PubMed] [Google Scholar]
- 24. Bahremand A., Nasrabady S. E., Ziai P., et al., “Involvement of Nitric Oxide‐cGMP Pathway in the Anticonvulsant Effects of Lithium Chloride on PTZ‐Induced Seizure in Mice,” Epilepsy Research 89, no. 2–3 (2010): 295–302, 10.1016/j.eplepsyres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 25. Manavi M. A., Toutounchian S., Afsahi S., Ebrahim Soltani Z., Mohammad Jafari R., and Dehpour A. R., “Ivermectin Exerts Anticonvulsant Effects Against Status Epilepticus Induced by Lithium‐Pilocarpine in Rats via GABA(A) Receptor and Neuroinflammation Modulation: Possible Interaction of Opioidergic Pathways and K(ATP) Channel With Nitrergic System,” Molecular Neurobiology 61, no. 10 (2024): 7627–7638, 10.1007/s12035-024-04061-3. [DOI] [PubMed] [Google Scholar]
- 26. Blei F., Fricke J., Wick J., Slot J. C., and Hoffmeister D., “Iterative l‐Tryptophan Methylation in Psilocybe Evolved by Subdomain Duplication,” Chembiochem 19, no. 20 (2018): 2160–2166, 10.1002/cbic.201800336. [DOI] [PubMed] [Google Scholar]
- 27. Park H. R. and Cai M., “Antiseizure Effects of Lilii Bulbus on Pentylenetetrazol Kindling‐Induced Seizures in Mice: Involvement of Reelin, Netrin‐1, and Semaphorin,” Biomedicine & Pharmacotherapy 173 (2024): 116385, 10.1016/j.biopha.2024.116385. [DOI] [PubMed] [Google Scholar]
- 28. Manavi M. A., Mohammad Jafari R., Shafaroodi H., Ejtemaei‐Mehr S., Sharifzadeh M., and Dehpour A. R., “Anticonvulsant Effects of Ivermectin on Pentylenetetrazole‐ and Maximal Electroshock‐Induced Seizures in Mice: The Role of GABAergic System and K(ATP) Channels,” Heliyon 8, no. 11 (2022): e11375, 10.1016/j.heliyon.2022.e11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Javaid U., Afroz S., Ashraf W., et al., “Ameliorative Effect of Nyctanthes Arbor‐Tristis L. by Suppression of Pentylenetetrazole‐Induced Kindling in Mice: An Insight From EEG, Neurobehavioral and In‐Silico Studies,” Biomedicine & Pharmacotherapy 175 (2024): 116791, 10.1016/j.biopha.2024.116791. [DOI] [PubMed] [Google Scholar]
- 30. Glatfelter G. C., Naeem M., Pham D. N. K., et al., “Receptor Binding Profiles for Tryptamine Psychedelics and Effects of 4‐Propionoxy‐N,N‐Dimethyltryptamine in Mice,” ACS Pharmacology & Translational Science 6, no. 4 (2023): 567–577, 10.1021/acsptsci.2c00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang J., Liang M., Shang Q., et al., “Psilocin Suppresses Methamphetamine‐Induced Hyperlocomotion and Acquisition of Conditioned Place Preference via D2R‐Mediated ERK Signaling,” CNS Neuroscience & Therapeutics 29, no. 3 (2023): 831–841, 10.1111/cns.14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomann J., Kolaczynska K. E., Stoeckmann O. V., et al., “In Vitro and In Vivo Metabolism of Psilocybin's Active Metabolite Psilocin,” Frontiers in Pharmacology 15 (2024): 1391689, 10.3389/fphar.2024.1391689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pourshadi N., Rahimi N., Ghasemi M., Faghir‐Ghanesefat H., Sharifzadeh M., and Dehpour A. R., “Anticonvulsant Effects of Thalidomide on Pentylenetetrazole‐Induced Seizure in Mice: A Role for Opioidergic and Nitrergic Transmissions,” Epilepsy Research 164 (2020): 106362, 10.1016/j.eplepsyres.2020.106362. [DOI] [PubMed] [Google Scholar]
- 34. Eslami F., Shayan M., Amanlou A., Rahimi N., Dejban P., and Dehpour A. R., “Pentylenetetrazole Preconditioning Attenuates Severity of Status Epilepticus Induced by Lithium‐Pilocarpine in Male Rats: Evaluation of Opioid/NMDA Receptors and Nitric Oxide Pathway,” Pharmacological Reports 74, no. 4 (2022): 602–613, 10.1007/s43440-022-00387-8. [DOI] [PubMed] [Google Scholar]
- 35. Moradi F., Eslami F., Rahimi N., et al., “Modafinil Exerts Anticonvulsive Effects Against Lithium‐Pilocarpine‐Induced Status Epilepticus in Rats: A Role for Tumor Necrosis Factor‐α and Nitric Oxide Signaling,” Epilepsy & Behavior 130 (2022): 108649, 10.1016/j.yebeh.2022.108649. [DOI] [PubMed] [Google Scholar]
- 36. Mumtaz F., Shafaroodi H., Nezamoleslami S. M., et al., “R.DehpourInvolvement of nNOS, and α1, α2, β1, and β2 Subunits of Soluble Guanylyl Cyclase Genes Expression in Anticonvulsant Effect of Sumatriptan on Pentylenetetrazole‐Induced Seizure in Mice,” Iranian Journal of Pharmaceutical Research 19, no. 4 (2020): 181–192, 10.22037/ijpr.2020.112594.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rahimian R., Khoshneviszadeh M., Bahremand T., Zirak M. R., Dehpour A. R., and Mousavizadeh K., “Oxytocinergic System Mediates the Proconvulsant Effects of Sildenafil: The Role of Calcineurin,” Hormones and Behavior 122 (2020): 104753, 10.1016/j.yhbeh.2020.104753. [DOI] [PubMed] [Google Scholar]
- 38. Afrooghe A., Babaei M., Shayan M., Ahmadi E., Mohammad Jafari R., and Dehpour A. R., “Therapeutic Potential of Bromhexine for Acute Itch in Mice: Involvement of TMPRSS2 and Kynurenine Pathway,” International Immunopharmacology 117 (2023): 109919, 10.1016/j.intimp.2023.109919. [DOI] [PubMed] [Google Scholar]
- 39. Shayesteh S., Guillemin G. J., Rashidian A., et al., “1‐Methyl Tryptophan, an Indoleamine 2,3‐Dioxygenase Inhibitor, Attenuates Cardiac and Hepatic Dysfunction in Rats With Biliary Cirrhosis,” European Journal of Pharmacology 908 (2021): 174309, 10.1016/j.ejphar.2021.174309. [DOI] [PubMed] [Google Scholar]
- 40. Aghili S. H., Manavi M. A., Panji M., Farhang Ranjbar M., Abrishami R., and Dehpour A. R., “Mirtazapine Improves Locomotor Activity and Attenuates Neuropathic Pain Following Spinal Cord Injury in Rats via Neuroinflammation Modulation,” Neurochemical Research 49, no. 12 (2024): 3326–3341, 10.1007/s11064-024-04240-7. [DOI] [PubMed] [Google Scholar]
- 41. Alexander S. P. H., Christopoulos A., Davenport A. P., et al., “The Concise Guide to PHARMACOLOGY 2023/24: G Protein‐Coupled Receptors,” British Journal of Pharmacology 180, no. Suppl 2 (2023): S23–S144, 10.1111/bph.16177. [DOI] [PubMed] [Google Scholar]
- 42. Harding S. D., Sharman J. L., Faccenda E., et al., “The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and Expansion to Encompass the New Guide to IMMUNOPHARMACOLOGY,” Nucleic Acids Research 46, no. D1 (2017): D1091–D1106, 10.1093/nar/gkx1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yuen E. S. and Trocóniz I. F., “Can Pentylenetetrazole and Maximal Electroshock Rodent Seizure Models Quantitatively Predict Antiepileptic Efficacy in Humans?,” Seizure 24 (2015): 21–27, 10.1016/j.seizure.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 44. Carhart‐Harris R. L., Bolstridge M., Day C. M. J., et al., “Psilocybin With Psychological Support for Treatment‐Resistant Depression: Six‐Month Follow‐Up,” Psychopharmacology 235, no. 2 (2018): 399–408, 10.1007/s00213-017-4771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fadahunsi N., Lund J., Breum A. W., et al., “Acute and Long‐Term Effects of Psilocybin on Energy Balance and Feeding Behavior in Mice,” Translational Psychiatry 12, no. 1 (2022): 330, 10.1038/s41398-022-02103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aday J. S., Mitzkovitz C. M., Bloesch E. K., Davoli C. C., and Davis A. K., “Long‐Term Effects of Psychedelic Drugs: A Systematic Review,” Neuroscience and Biobehavioral Reviews 113 (2020): 179–189, 10.1016/j.neubiorev.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 47. Zhao X., Du Y., Yao Y., et al., “Psilocybin Promotes Neuroplasticity and Induces Rapid and Sustained Antidepressant‐Like Effects in Mice,” Journal of Psychopharmacology 38, no. 5 (2024): 489–499, 10.1177/02698811241249436. [DOI] [PubMed] [Google Scholar]
- 48. Shafiee A., Arabzadeh Bahri R., Rafiei M. A., et al., “The Effect of Psychedelics on the Level of Brain‐Derived Neurotrophic Factor: A Systematic Review and Meta‐Analysis,” Journal of Psychopharmacology 38, no. 5 (2024): 425–431, 10.1177/02698811241234247. [DOI] [PubMed] [Google Scholar]
- 49. Lyons A., “Self‐Administration of Psilocybin in the Setting of Treatment‐Resistant Depression,” Innovations in Clinical Neuroscience 19, no. 7–9 (2022): 44–47. [PMC free article] [PubMed] [Google Scholar]
- 50. Gilliam F. G., Hecimovic H., and Gentry M. S., “Serotonergic Therapy in Epilepsy,” Current Opinion in Neurology 34, no. 2 (2021): 206–212, 10.1097/wco.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hasler G., Bonwetsch R., Giovacchini G., et al., “5‐HT1A Receptor Binding in Temporal Lobe Epilepsy Patients With and Without Major Depression,” Biological Psychiatry 62, no. 11 (2007): 1258–1264, 10.1016/j.biopsych.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. López‐Meraz M. L., González‐Trujano M. E., Neri‐Bazán L., Hong E., and Rocha L. L., “5‐HT1A Receptor Agonists Modify Epileptic Seizures in Three Experimental Models in Rats,” Neuropharmacology 49, no. 3 (2005): 367–375, 10.1016/j.neuropharm.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 53. Danober L., Deransart C., Depaulis A., Vergnes M., and Marescaux C., “Pathophysiological Mechanisms of Genetic Absence Epilepsy in the Rat,” Progress in Neurobiology 55, no. 1 (1998): 27–57, 10.1016/s0301-0082(97)00091-9. [DOI] [PubMed] [Google Scholar]
- 54. Madsen M. K., Fisher P. M., Burmester D., et al., “Psychedelic Effects of Psilocybin Correlate With Serotonin 2A Receptor Occupancy and Plasma Psilocin Levels,” Neuropsychopharmacology 44, no. 7 (2019): 1328–1334, 10.1038/s41386-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shahar O., Botvinnik A., Esh‐Zuntz N., et al., “Role of 5‐HT2A, 5‐HT2C, 5‐HT1A and TAAR1 Receptors in the Head Twitch Response Induced by 5‐Hydroxytryptophan and Psilocybin: Translational Implications,” International Journal of Molecular Sciences 23, no. 22 (2022): 14148, 10.3390/ijms232214148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harari R., Chatterjee I., Getselter D., and Elliott E., “Psilocybin Induces Acute Anxiety and Changes in Amygdalar Phosphopeptides Independently From the 5‐HT2A Receptor,” iScience 27, no. 5 (2024): 109686, 10.1016/j.isci.2024.109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Masoumi M., Manavi M. A., Mohammad Jafari R., et al., “Cannabidiol Anticonvulsant Effects Against Lithium‐Pilocarpine‐Induced Status Epilepticus in Male Rats Are Mediated by Neuroinflammation Modulation and Cannabinoids 1 (CB1), but Not CB2 and GABA(A) Receptors,” Cannabis and Cannabinoid Research 9, no. 3 (2024): 797–808, 10.1089/can.2023.0067. [DOI] [PubMed] [Google Scholar]
- 58. Luszczki J. J., Czuczwar P., Cioczek‐Czuczwar A., and Czuczwar S. J., “Arachidonyl‐2′‐Chloroethylamide, a Highly Selective Cannabinoid CB1 Receptor Agonist, Enhances the Anticonvulsant Action of Valproate in the Mouse Maximal Electroshock‐Induced Seizure Model,” European Journal of Pharmacology 547, no. 1–3 (2006): 65–74, 10.1016/j.ejphar.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 59. Lazarini‐Lopes W., da Silva‐Júnior R. M. P., Servilha‐Menezes G., Do Val‐da Silva R. A., and Garcia‐Cairasco N., “Cannabinoid Receptor Type 1 (CB1R) Expression in Limbic Brain Structures After Acute and Chronic Seizures in a Genetic Model of Epilepsy,” Frontiers in Behavioral Neuroscience 14 (2020): 602258, 10.3389/fnbeh.2020.602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liimatainen S., Lehtimäki K., Raitala A., et al., “Increased Indoleamine 2,3‐Dioxygenase (IDO) Activity in Idiopathic Generalized Epilepsy,” Epilepsy Research 94, no. 3 (2011): 206–212, 10.1016/j.eplepsyres.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 61. Deng N., Hu J., Hong Y., et al., “Indoleamine‐2,3‐Dioxygenase 1 Deficiency Suppresses Seizures in Epilepsy,” Frontiers in Cellular Neuroscience 15 (2021): 638854, 10.3389/fncel.2021.638854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xie W., Cai L., Yu Y., et al., “Activation of Brain Indoleamine 2,3‐Dioxygenase Contributes to Epilepsy‐Associated Depressive‐Like Behavior in Rats With Chronic Temporal Lobe Epilepsy,” Journal of Neuroinflammation 11, no. 1 (2014): 41, 10.1186/1742-2094-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Banach M., Piskorska B., Czuczwar S. J., and Borowicz K. K., “Nitric Oxide, Epileptic Seizures, and Action of Antiepileptic Drugs,” CNS & Neurological Disorders Drug Targets 10, no. 7 (2011): 808–819, 10.2174/187152711798072347. [DOI] [PubMed] [Google Scholar]
- 64. Keshavarzi M., Ghasemi M., Manavi M. A., Dehpour A. R., and Shafaroodi H., “Anticonvulsant Effects of Pentoxifylline on Seizures Induced by Pentylenetetrazole and Maximal Electroshock in Male Mice: The Role of the Nitrergic Pathway,” IBRO Neuroscience Reports 17 (2024): 485–492, 10.1016/j.ibneur.2024.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liy P. M., Puzi N. N. A., Jose S., and Vidyadaran S., “Nitric Oxide Modulation in Neuroinflammation and the Role of Mesenchymal Stem Cells,” Experimental Biology and Medicine (Maywood, N.J.) 246, no. 22 (2021): 2399–2406, 10.1177/1535370221997052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lesani A., Mashaknejadian Behbahani F., Manavi M. A., et al., “DehpourAcute Anticonvulsant Effects of Dapsone on PTZ‐ and MES‐Induced Seizures in Mice: NLRP3 Inflammasome Inhibition and Nrf2/HO‐1 Pathway Preservation,” Pharmacological Reports (2025), 10.1007/s43440-025-00698-6. [DOI] [PubMed] [Google Scholar]
- 67. Hammers A., Asselin M. C., Hinz R., et al., “Upregulation of Opioid Receptor Binding Following Spontaneous Epileptic Seizures,” Brain 130, no. 4 (2007): 1009–1016, 10.1093/brain/awm012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The data of the present study are available from the corresponding author on reasonable request.


