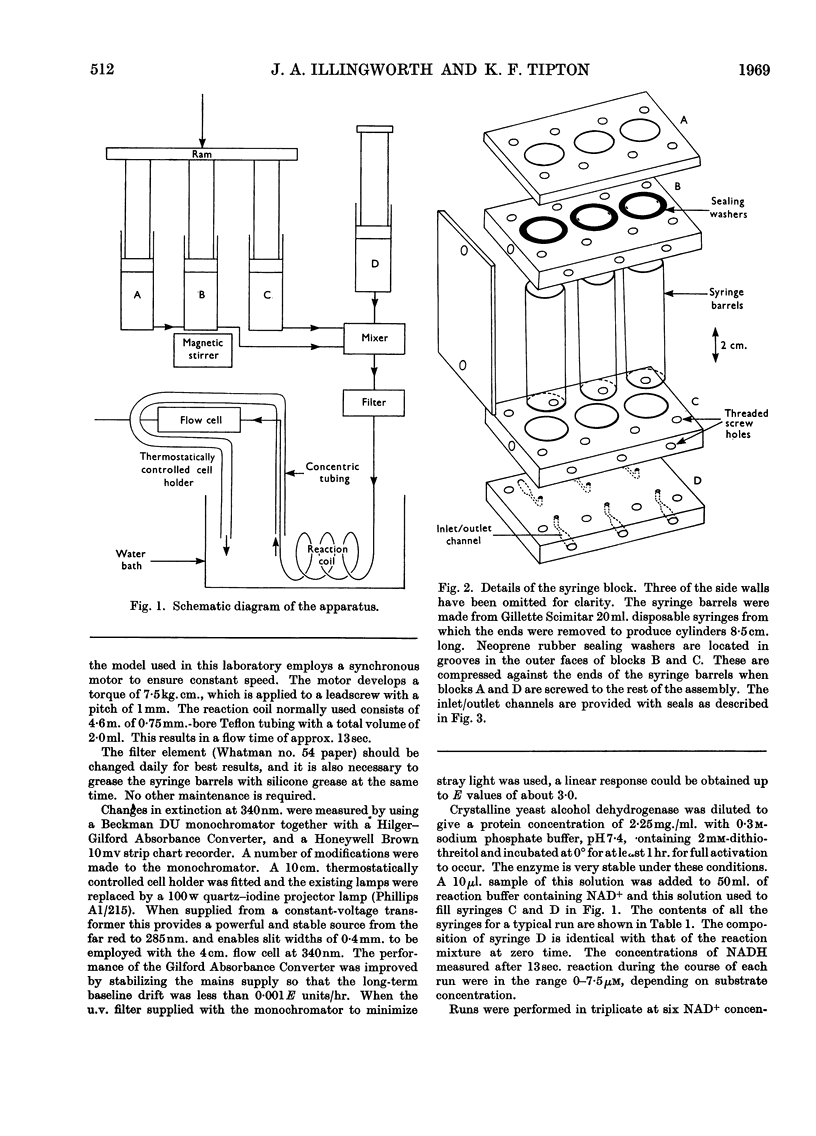
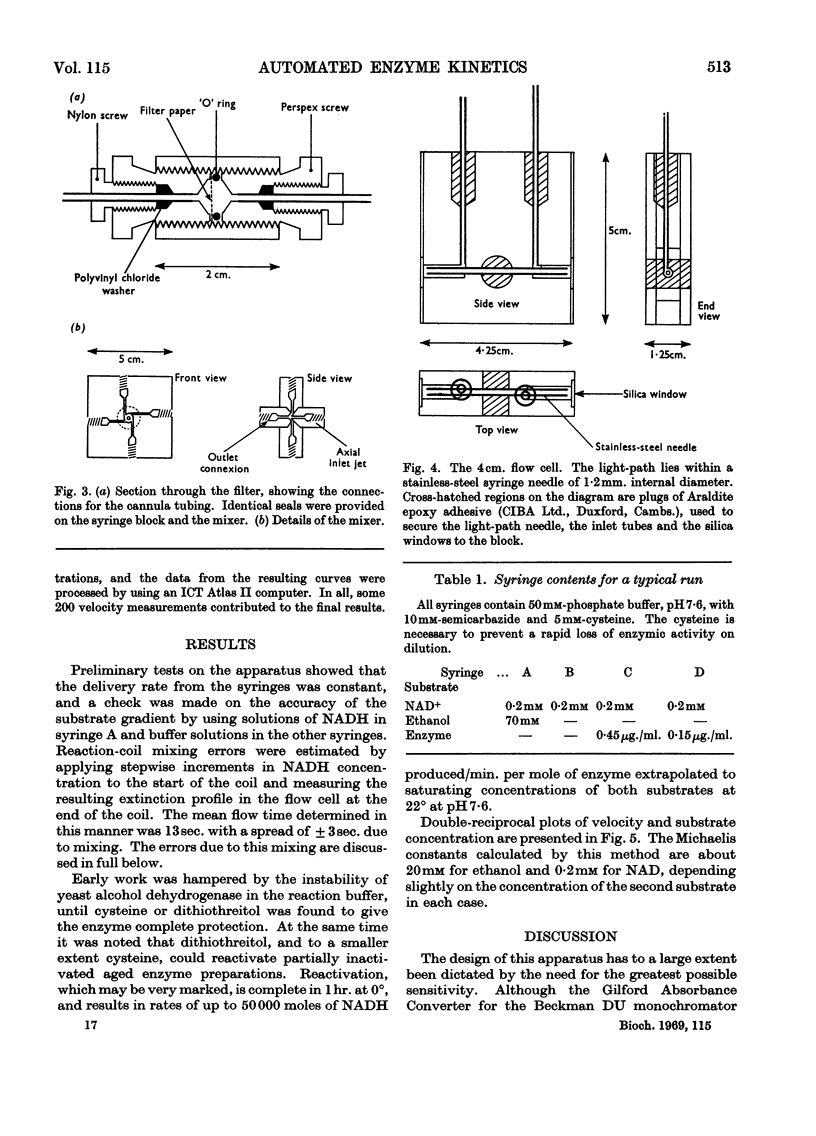
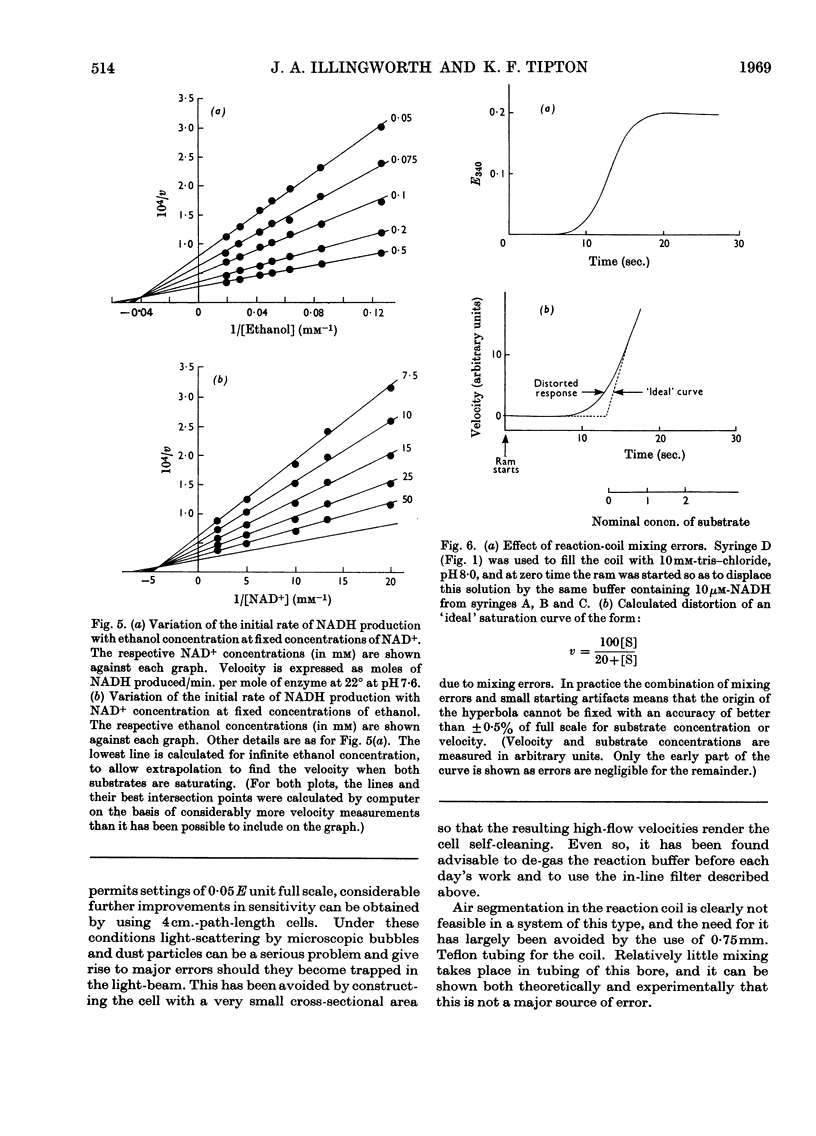
Abstract
A continuous-flow apparatus is described for automatically plotting substrate saturation curves, and is suitable for use with a variety of enzymes. A linear concentration gradient of the variable substrate is combined with a fixed proportion of the other substrates and the enzyme, and after passing through a reaction coil the product concentrations are measured spectrophotometrically. Use of a 4cm. flow cell and modified spectrophotometer permits accurate measurement of NADH concentration in the region of 0·1μm. Precise control over reaction times and substrate concentration is achieved by using power-driven syringes with an integral mixer. Specimen results are given for yeast alcohol dehydrogenase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HAYES J. E., Jr, VELICK S. F. Yeast alcohol dehydrogenase: molecular weight, coenzyme binding, and reaction equilibria. J Biol Chem. 1954 Mar;207(1):225–244. [PubMed] [Google Scholar]
- SCHWARTZ M. K., BODANSKY O. AUTOMATED METHODS FOR DETERMINATION OF ENZYME ACTIVITY. Methods Biochem Anal. 1963;11:211–246. doi: 10.1002/9780470110294.ch4. [DOI] [PubMed] [Google Scholar]
- Schwartz M. K., Bodansky O. Utilization of automation for studies of enzyme kinetics. Methods Biochem Anal. 1968;16:183–218. doi: 10.1002/9780470110348.ch3. [DOI] [PubMed] [Google Scholar]
- WOLFF H. P., TORBICA M. M. [Determination of loosely bound aldosterone in the blood of healthy and sick people]. Klin Wochenschr. 1963 Jan 1;41:40–42. doi: 10.1007/BF01478620. [DOI] [PubMed] [Google Scholar]


