Abstract
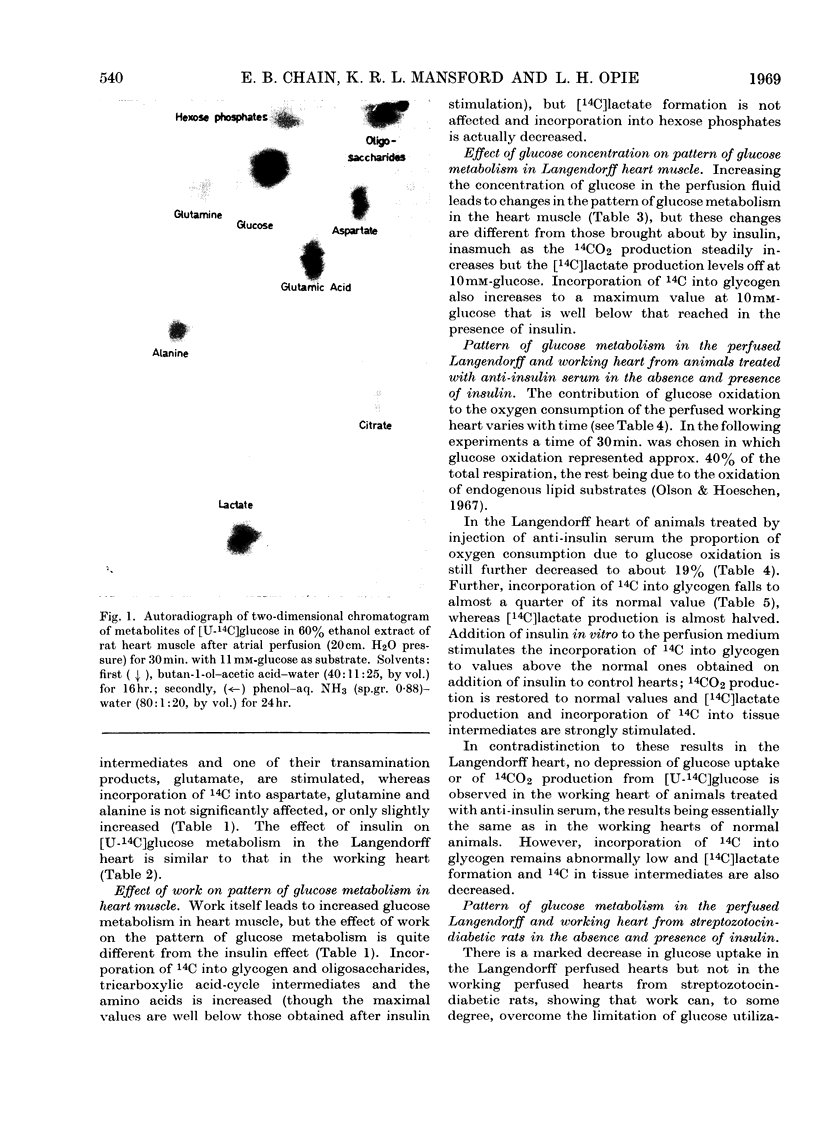
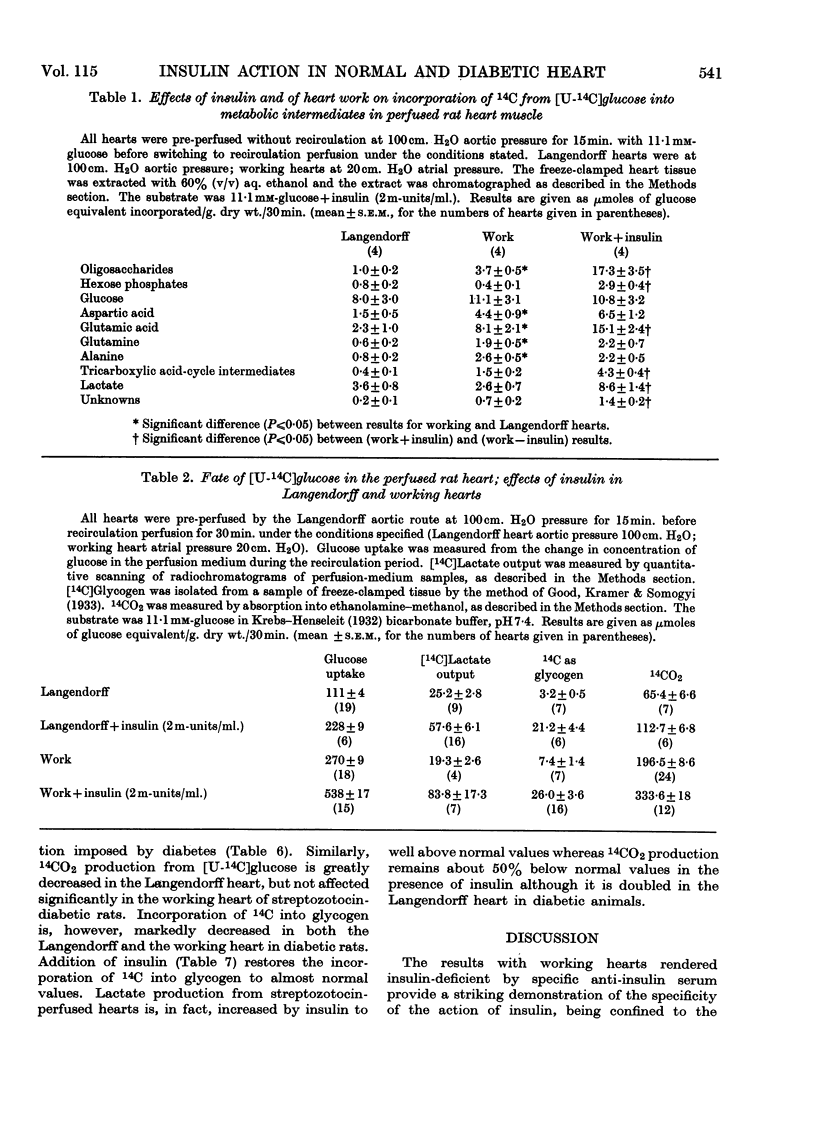
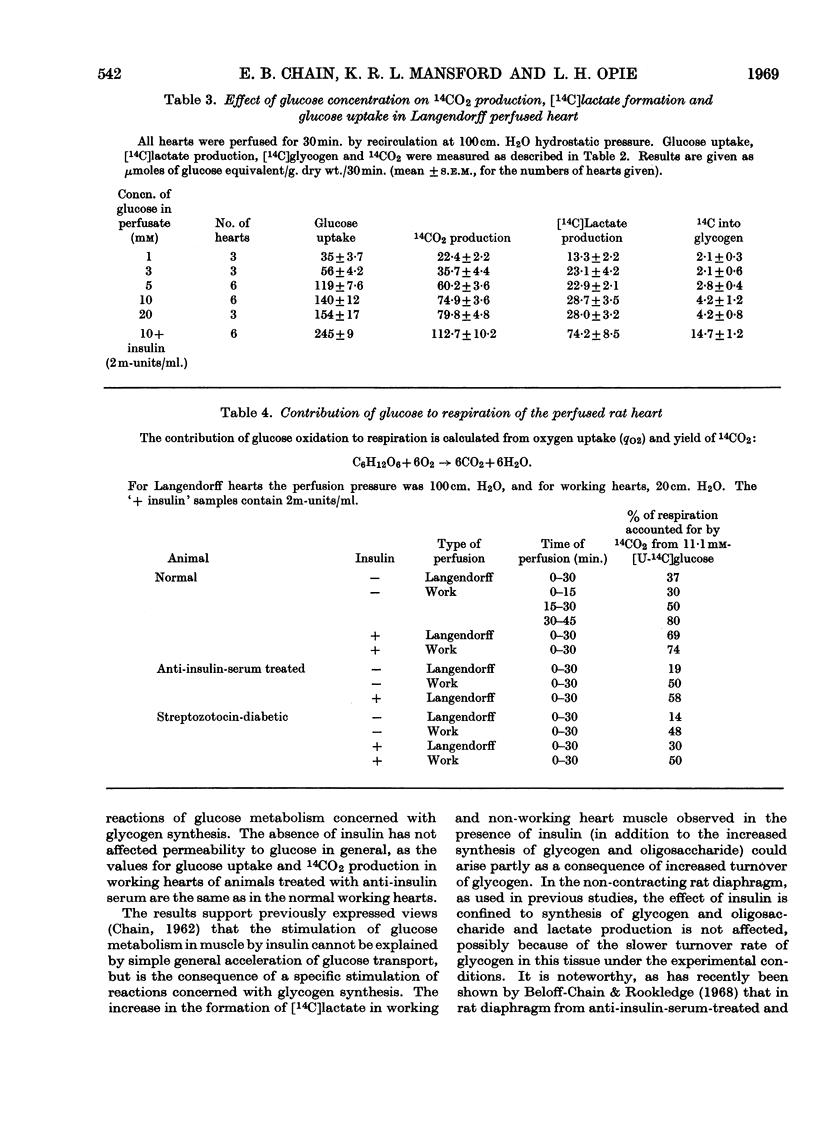
1. The metabolic pattern of [U-14C]glucose in the isolated rat heart has been studied, with both retrograde aortic (Langendorff) and atrially (working) perfused preparations in the presence and absence of insulin, in normal animals, animals rendered insulin-deficient (by injection of anti-insulin serum 1hr. before excision of the heart) and animals rendered diabetic by streptozotocin injection 7 days before use. 2. Radioautochromatograms of heart extracts show that the pattern of glucose metabolism in heart muscle is more complex than in diaphragm muscle. In addition to 14CO2, glycogen, oligosaccharides, phosphorylated sugars and lactate (the main metabolites formed from [14C]glucose in diaphragm muscle), 14C label from [14C]glucose appears in heart muscle in glutamate, glutamine, aspartate and alanine, and in tricarboxylic acid-cycle intermediates. 3. By a quantitative scanning technique of two-dimensional chromatograms it was found that a mechanical work load stimulates glucose metabolism, increasing by a factor of 2–3 incorporation of 14C into all the metabolites mentioned above except lactate and phosphorylated sugars, into which 14C incorporation is in fact diminished; 14CO2 production is equally stimulated. 4. Addition of insulin to the perfusion fluid of the working heart causes increases in 14C incorporation, by a factor of about 1·5 into 14CO2, by a factor of about 3–5 into glycogen, lactate and phosphorylated sugars, by a factor of about 2–3 into glutamate and tricarboxylic acid-cycle intermediates and by a factor of about 0·5 into aspartate, whereas incorporation into alanine and glutamine is not affected. The effect of a work load on the pattern of glucose metabolism is thus different from that of insulin. 5. Increasing the concentration of glucose in the perfusion fluid from 1 to 20mm leads to changes of the pattern of glucose metabolism different from that brought about by insulin. 14CO2 production steadily increases whereas [14C]lactate and glycogen production levels off at 10mm-glucose, at values well below those reached in the presence of insulin. 6. In Langendorff hearts of animals rendered insulin-deficient by anti-insulin serum or streptozotocin, glucose uptake, formation of 14CO2 and [14C]lactate, and 14C incorporation into glycogen and oligosaccharides are decreased. In insulin-deficient working hearts, however, glucose uptake and 14CO2 production are normal, whereas incorporation of 14C into glycogen and [14C]lactate production are greatly decreased. 7. Insulin added to the perfusion fluid restores 14C incorporation from glucose into 14CO2, glycogen and lactate in the Langendorff heart from animals rendered insulin-deficient by anti-insulin serum; in hearts from streptozotocin-diabetic animals addition of insulin restores 14C incorporation into glycogen and lactate, but 14CO2 production remains about 50% below normal. 8. The bearing of these results on the problem of the mode of action of insulin is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMIN J., GRANT R. T., WRIGHT P. H. Experimental diabetes in rats produced by parenteral administration of antiinsulin serum. J Physiol. 1960 Aug;153:146–165. doi: 10.1113/jphysiol.1960.sp006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELOFF-CHAIN A., CATANZARO R., CHAIN E. B., MASI I., POCCHIARI F., ROSSI C. The influence of insulin on carbohydrate metabolism in the isolated diaphragm muscle of normal and alloxan diabetic rats. Proc R Soc Lond B Biol Sci. 1955 May 17;143(913):481–503. doi: 10.1098/rspb.1955.0025. [DOI] [PubMed] [Google Scholar]
- BLEEHEN N. M., FISHER R. B. The action of insulin in the isolated rat heart. J Physiol. 1954 Feb 26;123(2):260–276. doi: 10.1113/jphysiol.1954.sp005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloff-Chain A., Betto P., Catanzaro R., Chain E. B., Longinotti L., Masi I., Pocchiari F. The metabolism of glucose 1-phosphate and glucose 6-phosphate and their influence on the metabolism of glucose in rat-diaphragm muscle. Biochem J. 1964 Jun;91(3):620–624. doi: 10.1042/bj0910620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloff-Chain A., Rookledge K. A. The metabolism of glucose in diaphragm muscle from normal rats, from streptozotocin-treated diabetic rats and from rats treated with anti-insulin serum. Biochem J. 1968 Dec;110(3):529–532. doi: 10.1042/bj1100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman S. P. A molecular basis for the mechanism of insulin action. Am J Med. 1966 May;40(5):740–749. doi: 10.1016/0002-9343(66)90155-0. [DOI] [PubMed] [Google Scholar]
- CROWLEY G. J., MOSES V., ULLRICH J. A VERSATILE SOLVENT TO REPLACE PHENOL FOR THE PAPER CHROMATOGRAPHY OF RADIOACTIVE INTERMEDIARY METABOLITES. J Chromatogr. 1963 Oct;12:219–228. doi: 10.1016/s0021-9673(01)83673-6. [DOI] [PubMed] [Google Scholar]
- DANFORTH W. H. GLYCOGEN SYNTHETASE ACTIVITY IN SKELETAL MUSCLE. INTERCONVERSION OF TWO FORMS AND CONTROL OF GLYCOGEN SYNTHESIS. J Biol Chem. 1965 Feb;240:588–593. [PubMed] [Google Scholar]
- GREGOR W. H., MARTIN J. M., WILLIAMSON J. R., LACY P. E., KIPNIS D. M. A study of the diabetic syndrome produced in rats by anti-insulin serum. Diabetes. 1963 Jan-Feb;12:73–81. doi: 10.2337/diab.12.1.73. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Hepp D., Challoner D. R., Williams R. H. Studies on the action of insulin in isolated adipose tissue cells. I. Stimulation of incorporation of 32P-labeled inorganic phosphate into mononucleotides in the absence of glucose. J Biol Chem. 1968 Aug 10;243(15):4020–4026. [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. III. The effect of insulin on pentose uptake by the diaphragm. J Biol Chem. 1957 Feb;224(2):681–693. [PubMed] [Google Scholar]
- Katzen H. M. The effect of diabetes and insulin in vivo and in vitro on a low Km form of hexokinase from various rat tissues. Biochem Biophys Res Commun. 1966 Aug 23;24(4):531–536. doi: 10.1016/0006-291x(66)90352-4. [DOI] [PubMed] [Google Scholar]
- Kundig W., Kundig F. D., Anderson B., Roseman S. Restoration of active transport of glycosides in Escherichia coli by a component of a phosphotransferase system. J Biol Chem. 1966 Jul 10;241(13):3243–3246. [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- Mansford K. R. Influence of anti-insulin serum on glucose metabolism. II. In the perfused heart. Diabetes. 1967 Jul;16(7):475–477. doi: 10.2337/diab.16.7.475. [DOI] [PubMed] [Google Scholar]
- Mansford K. R., Opie L. Comparison of metabolic abnormalities in diabetes mellitus induced by streptozotocin or by alloxan. Lancet. 1968 Mar 30;1(7544):670–671. doi: 10.1016/s0140-6736(68)92103-x. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Neely J. R., Wood R. E., Liébecq C., Liebermeister H., Park C. R. Factors affecting glucose transport in heart muscle and erythrocytes. Fed Proc. 1965 Sep-Oct;24(5):1040–1045. [PubMed] [Google Scholar]
- Neely J. R., Liebermeister H., Battersby E. J., Morgan H. E. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol. 1967 Apr;212(4):804–814. doi: 10.1152/ajplegacy.1967.212.4.804. [DOI] [PubMed] [Google Scholar]
- Olson R. E., Hoeschen R. J. Utilization of endogenous lipid by the isolated perfused rat heart. Biochem J. 1967 Jun;103(3):796–801. doi: 10.1042/bj1030796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAKIETEN N., RAKIETEN M. L., NADKARNI M. R. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep. 1963 May;29:91–98. [PubMed] [Google Scholar]
- WILLIAMSON J. R. Effects of insulin and diet on the metabolism of L-lactate and glucose by the perfused rat heart. Biochem J. 1962 May;83:377–383. doi: 10.1042/bj0830377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Walters E., McLean P. Effect of alloxan-diabetes and treatment with anti-insulin serum on pathways of glucose metabolism in lactating rat mammary gland. Biochem J. 1968 Sep;109(3):407–417. doi: 10.1042/bj1090407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters E., McLean P. Multiple forms of glucose-adenosine triphosphate phosphotransferase in rat mammary gland. Biochem J. 1967 Sep;104(3):778–783. doi: 10.1042/bj1040778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. J., Mayer S. E. Hormonal effects on glycogen metabolism in the rat heart in situ. Mol Pharmacol. 1966 Sep;2(5):454–464. [PubMed] [Google Scholar]