Abstract
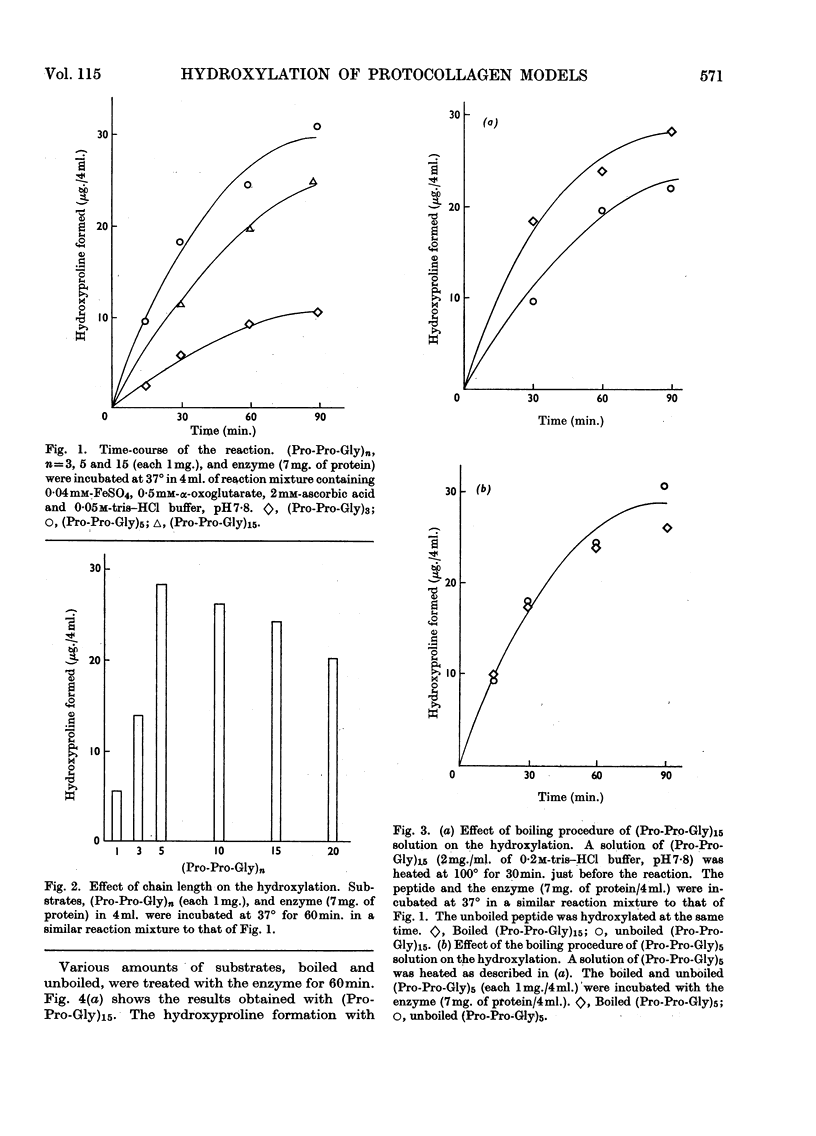
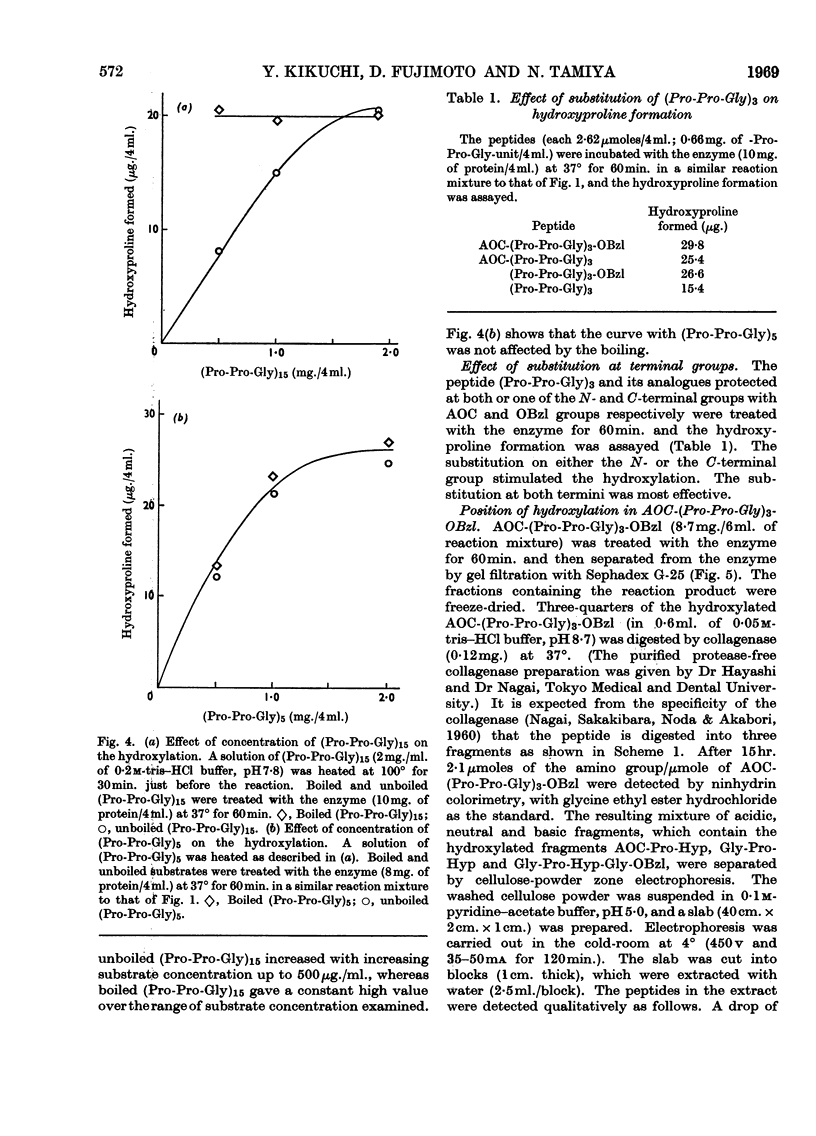
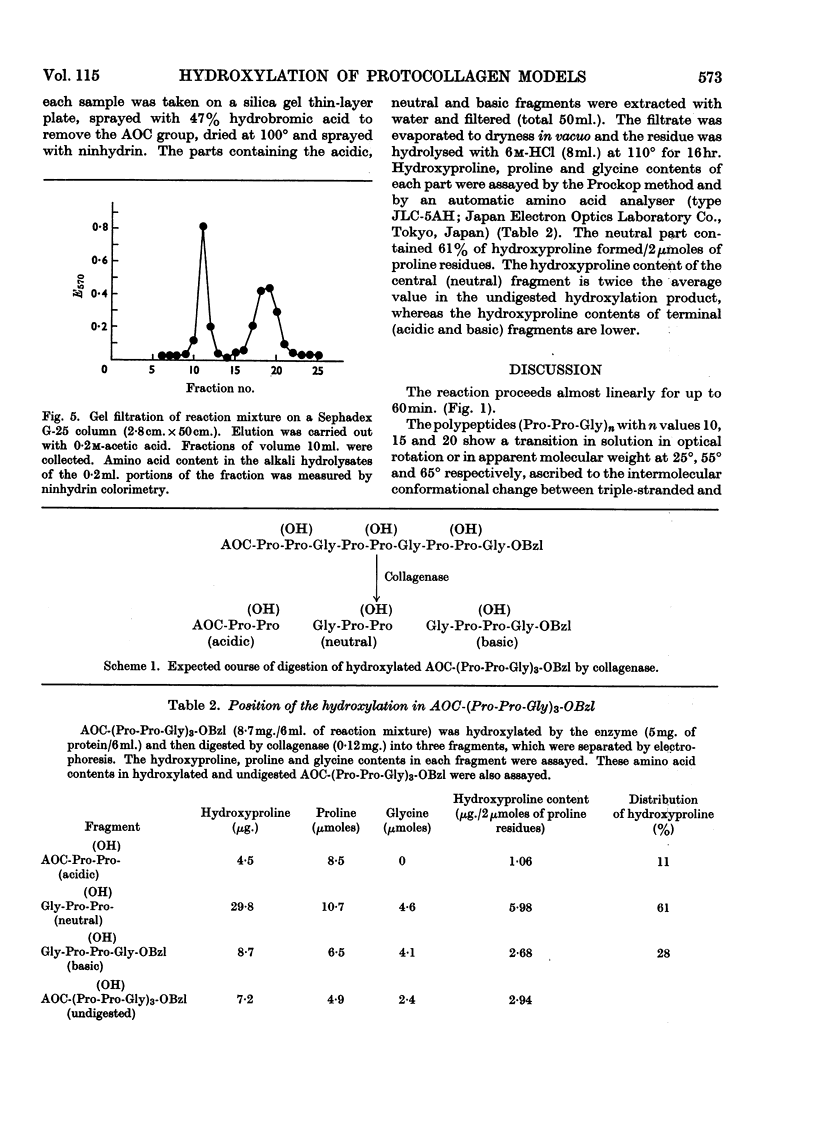
1. Synthetic polymers of l-prolyl-l-prolylglycine of defined chain length, (Pro-Pro-Gly)n, were found to be substrates for the enzyme protocollagen–proline hydroxylase, with optimum chain length n=5. Boiling the polymer (Pro-Pro-Gly)15 increased its activity as a substrate but had no effect on (Pro-Pro-Gly)5. 2. Protection of both or one of the N- and C-terminal groups made (Pro-Pro-Gly)3 a better substrate, and collagenase digestion of hydroxylated tert.-pentyloxy-carbonyl-(Pro-Pro-Gly)3 benzyl ester indicated that the central prolyl residues were the major points of hydroxylation. 3. The results suggest that the long-chain peptides are optimum substrates but that a triple-stranded structure is inhibitory for hydroxylation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Engel J., Kurtz J., Katchalski E., Berger A. Polymers tripeptides as collagen models. II. Conformational changes of poly(L-prolyl-glycyl-L-prolyl) in solution. J Mol Biol. 1966 May;17(1):255–272. doi: 10.1016/s0022-2836(66)80106-7. [DOI] [PubMed] [Google Scholar]
- Fujimoto D., Prockop D. J. Denatured collagen from the cuticle of Ascaris lumbricoides as a substrate for protocollagen proline hydroxylase. J Biol Chem. 1968 Aug 10;243(15):4138–4142. [PubMed] [Google Scholar]
- HARRINGTON W. F., VON HIPPEL P. H. The structure of collagen and gelatin. Adv Protein Chem. 1961;16:1–138. doi: 10.1016/s0065-3233(08)60028-5. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Fujimoto D., Tamiya N. Hydroxylation of poly(l-prolyl-l-prolylglycyl) of defined molecular weights by protocollagen proline hydroxylase. FEBS Lett. 1969 Feb;2(4):221–223. doi: 10.1016/0014-5793(69)80024-4. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Bright H. J., Prockop D. J. Kinetic patterns of protocollagen hydroxylase and further studies on the polypeptide substrate. Biochim Biophys Acta. 1968 Mar 25;151(3):558–567. doi: 10.1016/0005-2744(68)90002-8. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Hydroxylation of proline in synthetic polypeptides with purified protocollagen hydroxylase. J Biol Chem. 1967 Sep 25;242(18):4007–4012. [PubMed] [Google Scholar]
- MORITA K., HARUTA F. A SPRAYING METHOD FOR THE PREPARATION OF THIN-LAYER CHROMATOPLATES. J Chromatogr. 1963 Nov;12:412–412. doi: 10.1016/s0021-9673(01)83707-9. [DOI] [PubMed] [Google Scholar]
- NAGAI Y., SAKAKIBARA S., NODA H., AKABORI S. Hydrolysis of synthetic peptides by collagenase. Biochim Biophys Acta. 1960 Jan 29;37:567–569. doi: 10.1016/0006-3002(60)90531-x. [DOI] [PubMed] [Google Scholar]
- Nordwig A., Pfab F. K. Independence of collagen hydroxylation on the conformational state of the precursor substrate. Biochim Biophys Acta. 1968 Apr 9;154(3):603–605. doi: 10.1016/0005-2795(68)90026-3. [DOI] [PubMed] [Google Scholar]
- Sakakibara S., Inukai N. The trifluoroacetate method of peptide synthesis. II. An improved synthesis of bradykinin. Bull Chem Soc Jpn. 1966 Jul;39(7):1567–1572. doi: 10.1246/bcsj.39.1567. [DOI] [PubMed] [Google Scholar]
- Sakakibara S., Kishida Y., Nishizawa R., Shimonishi Y. Use of anhydrous hydrogen fluoride in peptide synthesis. II. Procedures for the syntheses of simple peptides. Bull Chem Soc Jpn. 1968 Feb;41(2):438–441. doi: 10.1246/bcsj.41.438. [DOI] [PubMed] [Google Scholar]
- Sakakibara S., Shimonishi Y., Kishida Y., Okada M., Sugihara H. Use of anhydrous hydrogen fluoride in peptide synthesis. I. Behavior of various protective groups in anhydrous hydrogen fluoride. Bull Chem Soc Jpn. 1967 Sep;40(9):2164–2167. doi: 10.1246/bcsj.40.2164. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Koyama E. Hydroxylation of proline in collagen model peptide. Biochim Biophys Acta. 1969 Feb 18;177(1):154–156. doi: 10.1016/0304-4165(69)90077-4. [DOI] [PubMed] [Google Scholar]


