Abstract
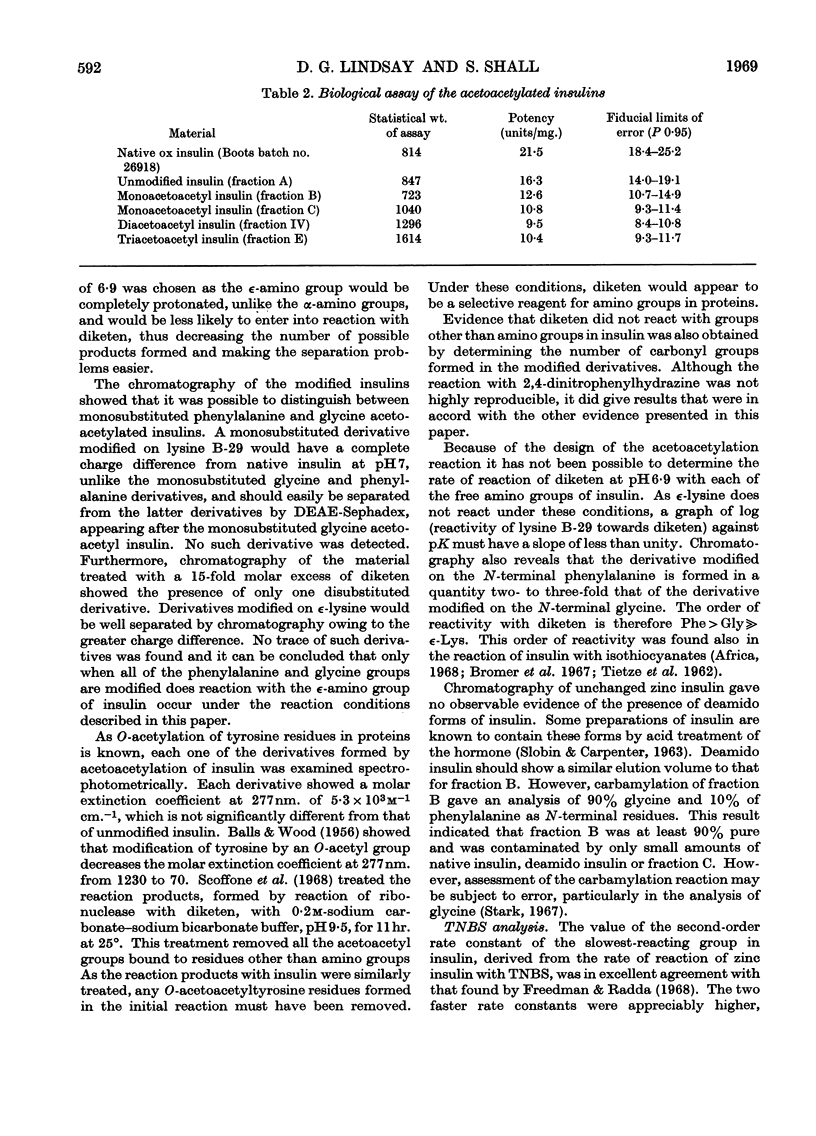
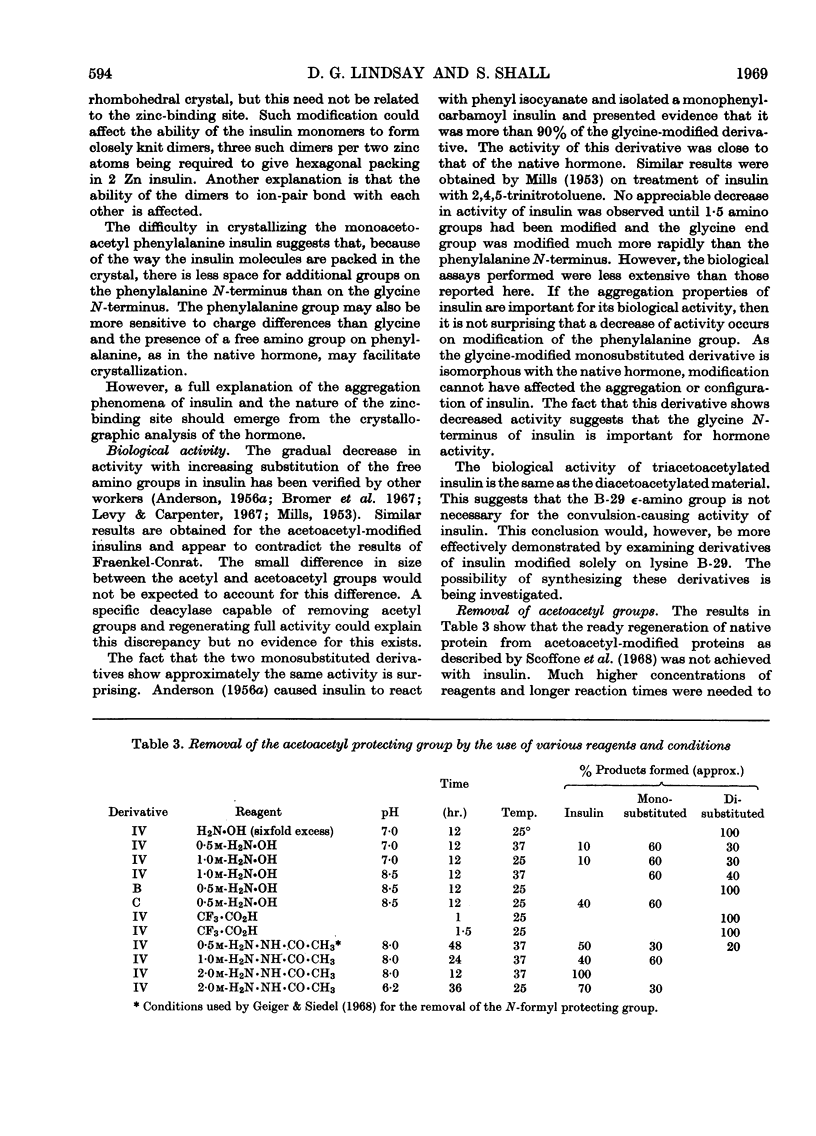
Insulin was treated with diketen at pH6·9. The reaction mixture was resolved into four components by DEAE-Sephadex chromatography. The first component was unchanged insulin. The second and third components were shown by end-group analysis to be substituted on phenylalanine B-1 and glycine A-1 respectively. The fourth component was disubstituted on both phenylalanine B-1 and glycine A-1. The ∈-amino group of lysine B-29 was not involved in the reaction at low reagent concentrations. The purity of these derivatives was checked by their electrophoretic behaviour and by measurement of the rate of their reaction with trinitrobenzenesulphonic acid. The hormonal activity of the derivatives was determined. The effect of the modifications on the hormonal activity and the tertiary structure of insulin is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLS A. K., WOOD H. N. Acetyl chymotrypsin and its reaction with ethanol. J Biol Chem. 1956 Mar;219(1):245–256. [PubMed] [Google Scholar]
- Brill A. S., Venable J. H., Jr The binding of transition metal ions in insulin crystals. J Mol Biol. 1968 Sep 28;36(3):343–353. doi: 10.1016/0022-2836(68)90160-5. [DOI] [PubMed] [Google Scholar]
- Bromer W. W., Chance R. E. Preparation and characterization of desoctapeptide-insulin. Biochim Biophys Acta. 1967 Feb 21;133(2):219–223. doi: 10.1016/0005-2795(67)90061-x. [DOI] [PubMed] [Google Scholar]
- Bromer W. W., Sheehan S. K., Berns A. W., Arquilla E. R. Preparation and properties of fluoresceinthiocarbamyl insulins. Biochemistry. 1967 Aug;6(8):2378–2388. doi: 10.1021/bi00860a013. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT J., FRAENKEL-CONRAT H. The essential groups of insulin. Biochim Biophys Acta. 1950 Mar;5(1):89–97. doi: 10.1016/0006-3002(50)90152-1. [DOI] [PubMed] [Google Scholar]
- Freedman R. B., Radda G. K. The reaction of 2,4,6-trinitrobenzenesulphonic acid with amino acids, Peptides and proteins. Biochem J. 1968 Jul;108(3):383–391. doi: 10.1042/bj1080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb A. R. A kinetic study of the reactions of amino acids and peptides with trinitrobenzenesulfonic acid. Biochemistry. 1966 Aug;5(8):2570–2574. doi: 10.1021/bi00872a013. [DOI] [PubMed] [Google Scholar]
- Graae J. The titration curve of insulin in the presence of various bivalent metal ions. Biochem J. 1968 Feb;106(4):777–781. doi: 10.1042/bj1060777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C. H. Preparation and properties of dinitrophenyl-NH(epsilon)-insulin. Nature. 1956 Dec 22;178(4547):1402–1402. doi: 10.1038/182001a0. [DOI] [PubMed] [Google Scholar]
- Levy D., Carpenter F. H. The synthesis of triaminoacyl-insulins and the use of the t-butyloxycarbonyl group for the reversible blocking of the amino groups of insulin. Biochemistry. 1967 Nov;6(11):3559–3568. doi: 10.1021/bi00863a030. [DOI] [PubMed] [Google Scholar]
- MILLS G. L. Observations on the composition and activity of partially arylated insulin. Biochem J. 1953 Jan;53(1):37–40. doi: 10.1042/bj0530037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- Marzotto A., Pajetta P., Galzigna L., Scoffone E. Reversible acetoacetylation of amino groups in proteins . Biochim Biophys Acta. 1968 Apr 9;154(3):450–456. doi: 10.1016/0005-2795(68)90004-4. [DOI] [PubMed] [Google Scholar]
- SLOBIN L. I., CARPENTER F. H. The labile amide in insulin: preparation of desalanine-desamido-insulin. Biochemistry. 1963 Jan-Feb;2:22–28. doi: 10.1021/bi00901a005. [DOI] [PubMed] [Google Scholar]
- TIETZE F., MORTIMORE G. E., LOMAX N. R. Preparation and properties of fluorescent insulin derivatives. Biochim Biophys Acta. 1962 May 21;59:336–346. doi: 10.1016/0006-3002(62)90182-8. [DOI] [PubMed] [Google Scholar]