Abstract
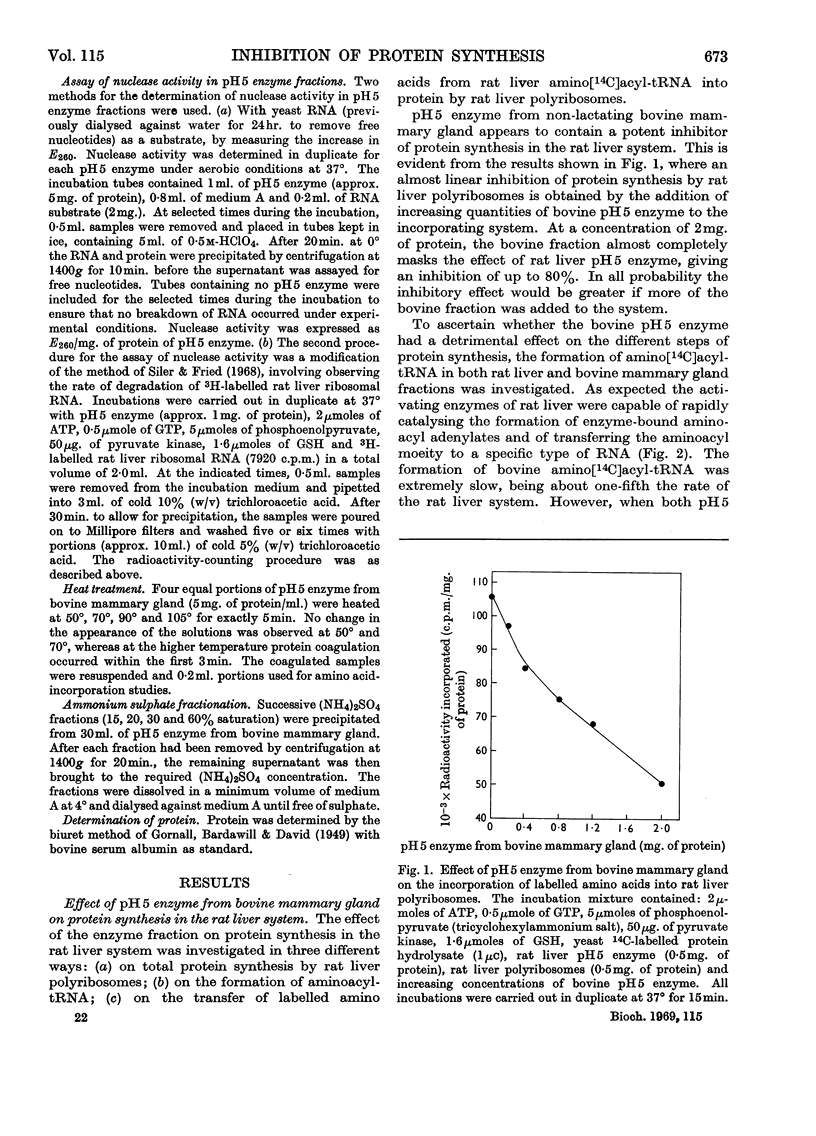
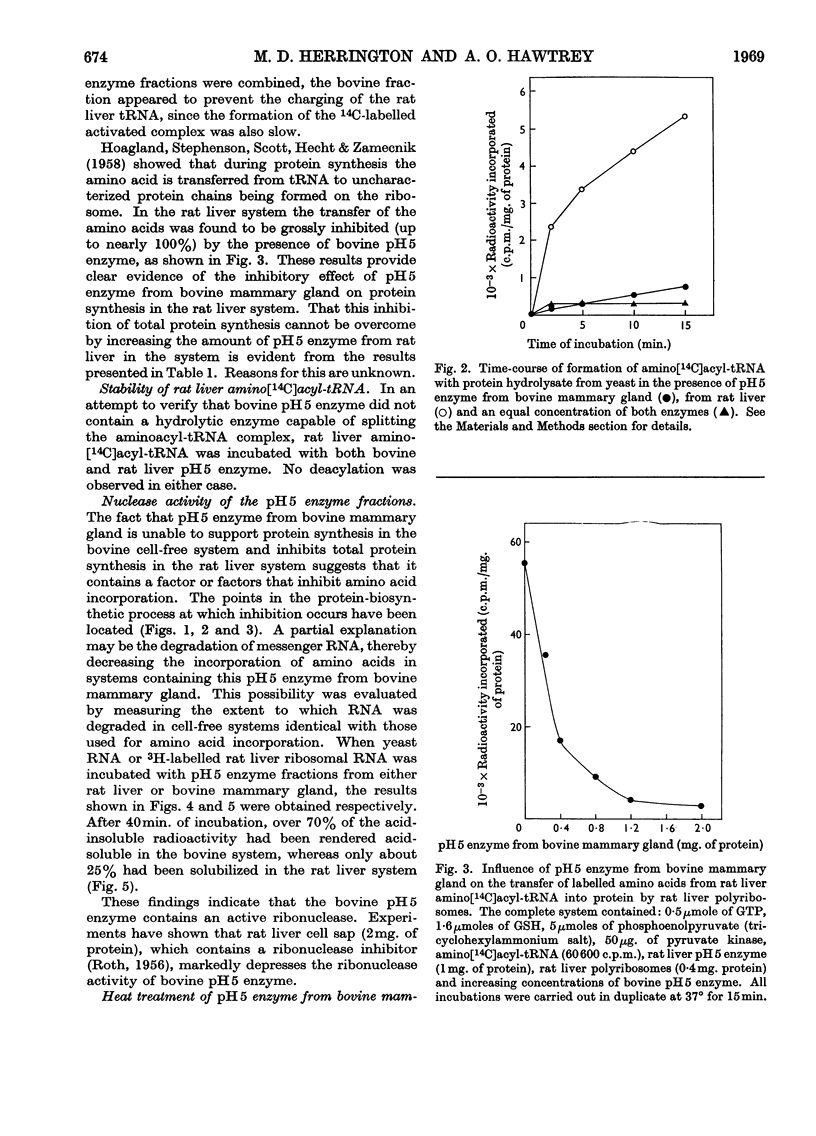
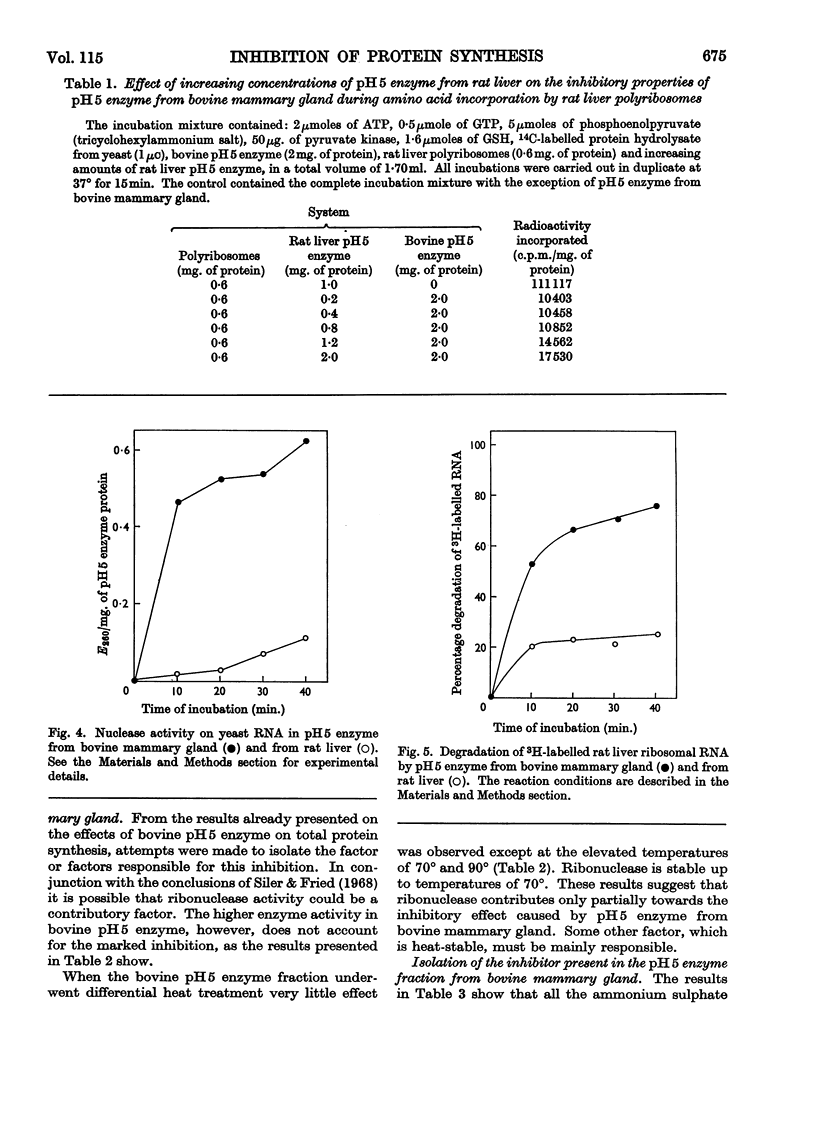
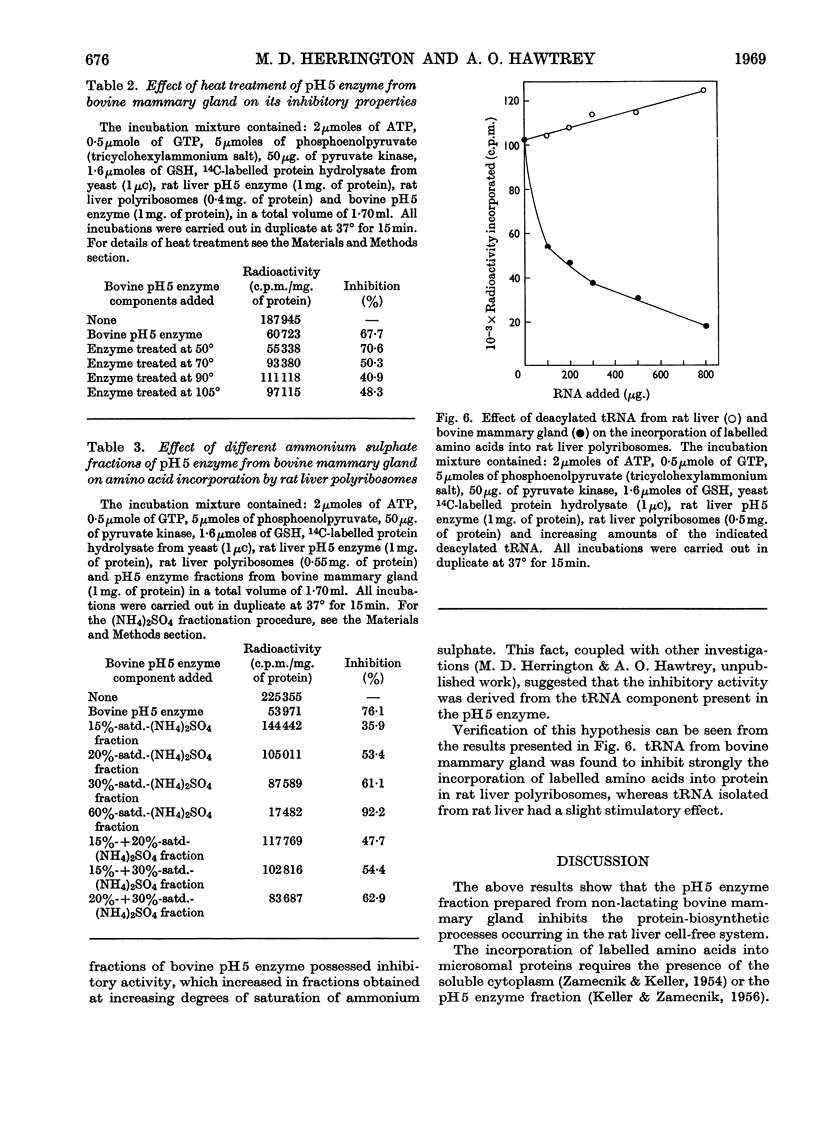
1. pH5 enzyme from non-lactating bovine mammary gland was found to contain potent inhibitors of protein synthesis in the rat liver cell-free system. These inhibitors affect (a) formation of aminoacyl-tRNA where tRNA represents transfer RNA, (b) transfer of labelled amino acids from rat liver amino[14C]acyl-tRNA to protein in rat liver polyribosomes, and (c) incorporation of 14C-labelled amino acids into peptide by rat liver polyribosomes supplemented with rat liver pH5 enzyme. 2. Increasing amounts of pH5 enzyme from bovine mammary gland progressively inhibited the incorporation of labelled amino acids into protein by a complete incorporating system from rat liver. Approx. 80% inhibition was observed at a concentration of 2mg. of protein of pH5 enzyme from bovine mammary gland. The inhibitory effect of the bovine pH5 enzyme fraction could not be overcome by the addition of increasing amounts of rat liver pH5 enzyme. 3. Fractionation of bovine pH5 enzyme with ammonium sulphate into four fractions showed that all the fractions inhibited the incorporation of 14C-labelled amino acids in the rat liver system, but to varying extents. The highest inhibition observed (90%) was exhibited by the 60%-saturated-ammonium sulphate fraction. 4. Heat treatment of bovine pH5 enzyme at various temperatures caused only a partial loss of its inhibitory effect on labelled amino acid incorporation by the rat liver system. Treatment at 105° for 5min. resulted in the bovine pH5 enzyme fraction losing 30% of its inhibitory activity. 5. pH5 enzyme from bovine mammary gland strongly inhibited the charging of rat liver tRNA in the presence of its own pH5 enzymes. 6. The transfer of labelled amino acids from rat liver amino[14C]acyl-tRNA to protein in a system containing rat liver polyribosomes and pH5 enzyme was almost completely inhibited by bovine pH5 enzyme at a concentration of 2mg. of protein of the enzyme fraction. 7. One of the inhibitors of various stages of protein synthesis in rat liver present in bovine pH5 enzyme was identified as an active ribonuclease, and the second inhibitor present was shown to be tRNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Korner A., Munro A. J. Inhibition by soluble ribonucleic acid of stimulatory effect of liver template ribonucleic acid. Biochem J. 1966 Nov;101(2):448–453. doi: 10.1042/bj1010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey N. H. The mechanism of protein synthesis in the developing chick embryo. The incorporation of free amino acids. Biochem J. 1964 May;91(2):335–340. doi: 10.1042/bj0910335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDLER R. W. Passage of radioactive amino acids through nonprotein fractions of hen oviduct during incorporation into protein. J Biol Chem. 1959 Jun;234(6):1466–1473. [PubMed] [Google Scholar]
- HOAGLAND M. B., KELLER E. B., ZAMECNIK P. C. Enzymatic carboxyl activation of amino acids. J Biol Chem. 1956 Jan;218(1):345–358. [PubMed] [Google Scholar]
- HOAGLAND M. B., STEPHENSON M. L., SCOTT J. F., HECHT L. I., ZAMECNIK P. C. A soluble ribonucleic acid intermediate in protein synthesis. J Biol Chem. 1958 Mar;231(1):241–257. [PubMed] [Google Scholar]
- HOAGLAND M. B., ZAMECNIK P. C., STEPHENSON M. L. Intermediate reactions in protein biosynthesis. Biochim Biophys Acta. 1957 Apr;24(1):215–216. doi: 10.1016/0006-3002(57)90175-0. [DOI] [PubMed] [Google Scholar]
- HUNTER G. D., GOODSALL R. A. Lipo-amino acid complexes from Bacillus megaterium and their possible role in protein synthesis. Biochem J. 1961 Mar;78:564–570. doi: 10.1042/bj0780564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawtrey A. O., Nourse L. D. The effect of 4-dimethylamino-3'-methylazobenzene on 14-C-labelled amino acid incorporation by rat-liver polysome preparations. Biochem J. 1966 Mar;98(3):682–688. doi: 10.1042/bj0980682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawtrey A. O., Schirren V., Dijkstra J. Studies on azo-dye carcinogenesis in rat liver. The effect of 4-dimethylamino-3'-methylazobenzene on the incorporation of [C]leucine into rat-liver microsomal protein. Biochem J. 1963 Jul;88(1):106–114. doi: 10.1042/bj0880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawtrey A. O. The effect of diisopropylfluorophosphate on [14C]-leucine incorporation by rat-liver ribosomes. S Afr J Med Sci. 1965 Dec;30(4):100–104. [PubMed] [Google Scholar]
- Heald P. J., Pohlman D. Amino acid incorporation by microsomal fractions from the liver of the domestic fowl. Biochim Biophys Acta. 1966 Aug 17;123(2):390–401. doi: 10.1016/0005-2787(66)90291-7. [DOI] [PubMed] [Google Scholar]
- Herrington M. D., Hawtrey A. O. Studies on subcellular fractions of non-lactating bovine mamm- ary gland. S Afr J Med Sci. 1969 Jul;34(2):49–58. [PubMed] [Google Scholar]
- KELLER E. B., ZAMECNIK P. C. The effect of guanosine diphosphate and triphosphate on the incorporation of labeled amino acids into proteins. J Biol Chem. 1956 Jul;221(1):45–59. [PubMed] [Google Scholar]
- SARIN P. S., ZAMECNIK P. C. ON THE STABILITY OF AMINOACYL-S-RNA TO NUCLEOPHILIC CATALYSIS. Biochim Biophys Acta. 1964 Dec 16;91:653–655. doi: 10.1016/0926-6550(64)90018-0. [DOI] [PubMed] [Google Scholar]
- Siler J. G., Fried M. Nuclease activity in cell-free amino acid-incorporating systems from chicken liver. Biochem J. 1968 Sep;109(2):185–190. doi: 10.1042/bj1090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]
- ZAMECNIK P. C., KELLER E. B. Relation between phosphate energy donors and incorporation of labeled amino acids into proteins. J Biol Chem. 1954 Jul;209(1):337–354. [PubMed] [Google Scholar]
- von der DECKEN, CAMPBELL P. N. Amino acid transfer from aminoacyl-ribonucleic acid to serum albumin by the microsome fraction from rat liver. Biochem J. 1962 Mar;82:448–454. doi: 10.1042/bj0820448. [DOI] [PMC free article] [PubMed] [Google Scholar]


