Abstract
With the continued exploration of the universe, there is an increasingly urgent need to address the health challenges arising from spaceflight. In space, astronauts are exposed to radiation, confinement and isolation, circadian rhythm dysregulation, and microgravity conditions that are different from those on Earth. These risk factors jeopardize astronauts’ health, thus affecting the quality of space missions. Among these factors, gravitational changes influence the balance between oxidation and antioxidants, stimulating the production of reactive oxygen species (ROS), finally leading to oxidative stress (OS). OS leads to oxidative damage of biomolecules such as lipids, proteins, and DNA, which causes the development of various diseases. The occurrence of OS is increased in microgravity and affects multiple systems, including the musculoskeletal, cardiovascular, nervous, and immune systems. In this review, we discuss the mechanisms of OS, the physiological effects on different systems caused by OS in microgravity environment, and potential treatments for OS. Finally, treatment strategies for oxidative stress in microgravity are summarized, providing some promising approaches for protecting the health of astronauts in future space exploration.
Keywords: oxidative stress, microgravity, reactive oxygen species, antioxidants, therapy
1. Introduction
Since the first human spaceflight, the exploration of the universe has never stopped, and the number of astronauts, the frequency of flights, and the duration of their missions are gradually increasing. Resolving health issues during spaceflights is becoming an urgent priority. In space, astronauts are exposed to space radiation, microgravity and altered gravity, confinement and isolation, hostile and closed environments, medical limitations, and other spaceflight risk factors. These factors compromise the health of astronauts, thus affecting the quality of space missions [1]. Among these factors, microgravity plays an important role that cannot be ignored. It causes structural and functional changes in the musculoskeletal, nervous, cardiovascular, digestive, and immune systems, among others, exposing astronauts to a range of health risks. In microgravity, space adaptation syndrome (SAS) is triggered in the early stages [2]. This syndrome can trigger symptoms such as nausea, disorientation, and generalized malaise, which typically last up to 72 h [3]. Due to the lack of downward attraction from gravity, body fluids are transferred to the upper body and head, increasing intracranial pressure (ICP) or optic nerve compression in the eyes [4]. This leads to retinal endothelium dysfunction, in turn causing several ophthalmic symptoms, including choroidal folds, optic disc edema, and cotton-wool spots [5]. This phenomenon has been summarized by Sara et al. as spaceflight-associated neuro-ocular syndrome (SANS) [6]. The transfer of body fluids to the head causes changes in the brain white matter microstructure [7], developing a hydrocephalus-like structure, which damages brain tissue and impairs brain function [8]. The microgravity environment also impairs bones and skeletal muscles, contributing to conditions such as osteoporosis and skeletal muscle atrophy [9,10]. In microgravity, the gut microbiota is dysregulated, the intestinal mucosal barrier is compromised, and mucosal permeability is increased [11]. As a result, the stability of the intestinal microenvironment and gastrointestinal dynamics is compromised, triggering local or systemic reactions [12]. The immune system function is also disrupted under the influence of microgravity, leading to phenomena such as an inhibition of T-cell antigen reactivity and a rise in white blood cell counts, neutrophils, and monocytes [13,14]. Currently, there are limited studies related to the molecular mechanisms involved in these symptoms caused by microgravity.
Exogenous risk factors such as radiation, tobacco, and drugs disrupt the balance between oxidation and antioxidants, stimulating the production of reactive oxygen species (ROS) and finally leading to oxidative stress (OS) [15]. Moreover, OS occurs in a variety of organs and cells, causing tissue damage and dysfunction in microgravity. Microgravity promotes the expression of oxidative enzymes, such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) [16]. NOXs expression is increased in the brain, skeletal muscles, blood vessels, and lungs in microgravity [17,18,19,20]. Microgravity also reduces the expression of antioxidant proteins, such as nuclear transcription factor red lineage 2-related factor 2 (Nrf2), which increases oxidative products and leads to OS [21]. Nrf2, a transcription factor, is essential to the OS response, as it binds to antioxidant response elements (AREs) and regulates antioxidant proteins [22]. Nrf2 abundance was reduced significantly in the flounder muscle of rats by using hindlimb unloading (HLU) to simulate microgravity, leading to muscle atrophy [19]. Although microgravity-induced OS causes difficulties for astronauts in space operations, the molecular mechanisms and treatments of OS in microgravity have been less well investigated.
This review summarizes the physiological changes in systems caused by OS in the microgravity environment, describes the treatments of OS both on the ground and in microgravity, and elucidates the health effects of OS in the microgravity environment. It provides some new ideas for future research on OS and for treating organ damage caused by OS in space in the future. This review provides a preliminary perspective on OS in space for the field, thereby laying the foundation for more focused future research. We collected relevant references through an extensive literature search to improve the comprehensiveness and authority of this review. Future research needs to explore more specific aspects to address the gaps identified in the current study.
2. Oxidative Stress
2.1. Mechanisms of Oxidative Stress
OS was first described in a study in 1985 [23]. OS occurs when the production and accumulation of ROS exceed the capacity of the antioxidant defense, resulting in a disruption of the metabolic homeostasis of the organism [24]. OS is characterized by a disruption of the oxidant–antioxidant balance. ROS, including superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH), lead to oxidative damage to lipids, proteins, and DNA in cells, triggering tissue damage [25]. To illustrate this, DNA damage was caused by the ROS-induced oxidation of guanine to pre-mutagenic 8-oxo-7,8-dihydroguanine (8-oxoG), which resulted in double-strand breaks during replication; proteins were misfolded due to exposure to ROS, causing changes in intramolecular interactions [26].
The production of ROS involves various factors. Mitochondria produce ROS during oxidative phosphorylation (OXPHOS) [27]. Similarly, enzymes such as NADPH oxidases (NOXs) also contribute to ROS production [28]. Additionally, ROS levels increased when the body underwent pathological changes, such as inflammatory reactions and ischemia/reperfusion injury [29,30]. Exogenous risk factors, including environmental pollutants, radiation, drugs, and tobacco, also directly or indirectly increased ROS production [15].
As well as increased ROS production, ROS accumulation due to an unregulated antioxidant system is also an important cause of OS. Antioxidant enzymes, metal ion chelators, small-molecule antioxidant compounds, and enzymes that play a role in repairing oxidative damage are all important components of the antioxidant system [25]. Antioxidant enzymes include superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX) [31]. Typically, antioxidant enzymes are activated through Nrf2 and PGC-1α signaling pathways [22]. Metal ion chelators bind to transition metal ions, thereby reducing the generation of free radicals catalyzed by these ions. For instance, ferritin and transferrin store and transport iron ions, preventing these ions from catalyzing oxidative reactions [32]. Furthermore, small-molecule antioxidant compounds, including ascorbic acid (vitamin C), alpha-tocopherol (vitamin E), glutathione (GSH), carotenoids, and flavonoid analogs, directly reacted with ROS to scavenge free radicals [33]. DNA damage repair-related enzymes, such as formamidopyrimidine DNA glycosylase (FPG) and 8-oxoguanine DNA glycosylase (OGG1), repair damage caused by OS [34]. In conclusion, the dysregulation of the antioxidant system results in the inability to rapidly scavenge ROS and repair damage from OS in a timely manner, leading to the development of diseases in organisms.
2.2. Effects of OS on the Ground
OS is a key factor in the development of various diseases on the ground. In some diseases, OS is a major factor in pathologies such as radiation-induced lung injury, paraquat poisoning, and atherosclerosis [25]. For instance, in a radiation environment, water molecules produced •OH, which led to an increase in oxidative intermediates, inflammation, and, ultimately, lung injury [35]. In the majority of cases, OS is an indirect factor in disease progression. The excessive production of ROS inhibits cell proliferation; accelerates cellular senescence; and induces senescence-related diseases, including retinal diseases, neurodegenerative diseases, osteoporosis, cardiovascular diseases, and cancers [36,37,38,39,40]. Moreover, ROS accumulation in the bone microenvironment was found to play a role in osteoblast and osteoclast apoptosis [41]. Research has also shown that ROS was increased in the rheumatoid arthritis-associated synovial microenvironment under hypoxia. A high-ROS-level environment caused fibroblast proliferation and the secretion of inflammatory factors by macrophage-like synoviocytes and increased angiogenesis, leading to cellular and tissue damage, driving disease progression [42]. In addition, a close interaction was found between OS and the immune response. ROS activated the immune response and promoted the release of cytokines, while immune cells produced ROS when clearing pathogens, which killed part of them [43]. However, excessive ROS inhibited immune activity and suppressed signal transduction between dendritic cells (DCs) and T cells, affecting the normal function of the immune system [44,45]. Matsushita et al. found that the lipid peroxidation of T cells induced iron death and prevented the immune response to infection [46]. In brief, on the ground, OS plays a crucial role in diseases by directly or indirectly affecting various organs and systems on the ground.
3. Effects of OS on the Organism in Microgravity
This section summarizes in vitro and in vivo experimental data from studies about microgravity to describe the effects of microgravity-induced OS on biological samples. Experiments in real microgravity environments are typically carried out during spaceflight, such as on rockets, on the ISS, and in parabolic flight [47]. However, experiments in space are costly, and the duration is limited by the work schedules. Therefore, experiments in simulated microgravity on the ground have become an important method [48]. Both in vivo and in vitro models have been available to simulate microgravity for experimental protocols. Among the in vivo models are HLU, long-duration bed rest, and dry immersion [49,50,51]. Additionally, in vitro models such as rotating wall vessels, the random positioning machine, and the 3D clinostat are also available for use [52]. These methods have various advantages and limitations, and each method has its unique value, which will not be elaborated upon here. However, different methods introduce variability in the experimental results. Therefore, we have listed the models summarizing the experimental data in tables.
3.1. Effects of OS on the Musculoskeletal System in Microgravity
Microgravity has a significant impact on the musculoskeletal system. On the ground, bones are stimulated by mechanical loads, new bone is generated to replace old or damaged bones, and bones are constantly remodeled, creating a balance [53]. This balance is broken by microgravity, which sharply reduces the mechanical loads on bones and muscles. New bone production slows down due to lack of stimulation, leading to bone loss [10,54]. Microgravity regulates the distribution and content of minerals in the body in many ways, such as altering the absorption and excretion processes of calcium and magnesium and changing the electrolyte balance [55]. The effect of changing the electrolyte balance on skeletal muscles is significant, including impaired muscle contraction and reduced muscular endurance and performance [56,57]. According to current studies, microgravity-induced OS leads to bone loss and skeletal muscle atrophy in the musculoskeletal system (Table 1).
Table 1.
Overview of the effects of OS on the musculoskeletal system.
| Type of Tissues/Cells | Model | Study Result | Refs. |
|---|---|---|---|
| Femur MC3T3-E1 cells |
HLU RCCS |
Cause bone loss by stimulating the Nrf2/HO-1 pathway | [58] |
| Femur | HLU | Alter bone microstructure and mechanical strength | [59] |
| Tibiae; Femur MC3T3-E1; RAW264.7 cells |
HLU RWV |
Reduce bone density and destroy bone structure in tibial, destroy mechanical strength in femur, reduce osteoblastic differentiation, and increase osteoclastogenesis | [60] |
| Human primary osteoblasts | RPM | Impair mitochondrial physiology as well as osteoblast function | [61] |
| Femur; lumbar vertebra MC3T3-E1; RAW264.7 cells |
HLU RWV |
Reduce bone mineral density, ultimate load, stiffness, and energy in femur and lumbar vertebra; reduce osteoblastic differentiation in MC3T3-E1 cells; induce osteoclastic differentiation and osteoclastogenesis in RAW264.7 cells | [62] |
| MC3T3-E1 cells | RPM | Alter cell cytoskeletal architecture, suppress cell proliferation rate and metabolism | [63] |
| Rat calvarial osteoblasts | RPM | Cause mitochondrial dysfunction and Ca2+ overload | [64] |
| Left vastus lateralis muscle | Unilateral lower limb suspension | Increase the mRNA expression of HMOX | [65] |
| L6 cells | ISS 3D clinostat |
Cause muscle atrophy through Cbl-b expression activated by the ERK-Egr signaling pathway | [66] |
| H9c2 cells | ISS | Damage DNA via down regulation of H2afx expression | [67] |
| Gastrocnemius and soleus muscles | HLU | Cause skeletal muscle atrophy and impair mitochondrial energetics | [68] |
| Soleus muscles | HLU | Induced soleus muscle atrophy via elevation of Nox2 | [19] |
| Quadriceps and soleus muscles | FLT | Decrease muscles wet weights | [69] |
HLU: hindlimb unloading; ISS: International Staging System; RCCS: Rotating Cell Culture System; RPM: random positioning machine; RWV: the rotating wall vessels; FLT: flight.
Microgravity-induced OS causes bone loss [58]. Lan et al. discovered that microgravity-induced OS caused an imbalance of bone metabolism, resulting in inhibited bone formation and enhanced bone resorption [59]. Microgravity-induced OS in the femur, evidenced by an increase in malondialdehyde (MDA) content and a decrease in total protein sulfhydryl content in the femur, leads to bone loss [60]. In vitro, microgravity also induced OS in osteoblasts, shown by a significant reduction in oxidized GSH and antioxidant enzymes, which reduced osteoblast differentiation and increased osteoclast formation [61]. Sun et al. reached the same conclusion [62]. Morabito et al. found that OS induced the impairment of the cytoskeleton structure, cell proliferation, and metabolism in osteoblasts [63]. Additionally, microgravity-induced OS caused the shortening of the primary cilia in osteoblasts. The injury on primary cilia led to increased expression and overactive channel of transient receptor potential vanilloid 4 (TRPV4), causing intracellular Ca2+ overload and OS, which eventually caused bone loss [64]. In summary, microgravity-induced OS disrupts bone metabolic homeostasis by decreasing osteoblast proliferation and differentiation, impairing cytoskeletal structure and metabolism, shortening the primary cilia of osteoblasts, and increasing osteoclast formation, which ultimately leads to bone loss.
Microgravity-induced OS causes skeletal muscle atrophy. The accumulation of monoubiquitinated lactate dehydrogenase (LDH) was found in muscles under simulated microgravity and spaceflight conditions in 2005. H2O2 induced the mono-ubiquitination of LDH-A in cells overexpressing LDH-A and ubiquitin, suggesting that simulated microgravity induced OS in skeletal muscles [70]. Biopsy samples of the vastus lateralis were collected after 48h unloading via unilateral lower limb suspension, and the OS response pathway was found to be the second-highest-ranked upregulated classical pathway [65]. A significant change was observed in flounder muscle in microgravity. The levels of antioxidant proteins (Gpx3, Gstm1, Gstm2, and Sod1) in flounder muscles were increased in spaceflight mice, suggesting that the disruption of antioxidant function contributed to OS in atrophied muscles [71]. Uchida et al. discovered that high levels ROS induced muscle atrophy via casitas B-lineage lymphoma-b (Cbl-b) expression activated by the ERK1/2 early growth response protein (Egr)-Cbl-b signaling pathway in microgravity [66]. Genchi et al., by sequencing muscle cells, found that H2A histone family member X (H2AFX), which is involved in many DNA damage metabolic pathways, was downregulated in microgravity [67,72]. The lack of H2AFX led to impaired redox homeostasis [73]. A number of studies on muscle atrophy due to microgravity-induced OS focused on the production of Nox2 and the reduction in Nrf2 levels. Mechanical unloading during microgravity led to skeletal muscle atrophy via increased ROS production by Nox2, as well as an impaired antioxidant system, affecting, e.g., sirtuin 1 (SIRT1) and SOD [68]. Nrf2 regulated antioxidant proteins, its expression was significantly reduced in microgravity, and the inhibition of Nox2 prevented the reduction in Nrf2 levels. The Nox2 inhibitor (gp91ds-tat) attenuated the unloading-induced increase in the p67phox subunit from Nox2, thereby attenuating the unloading-induced atrophy of halibut muscles [19]. In addition, computer prediction and RNA from the quadriceps muscles of spaceflight mice showed that the inhibition of integrin and Nrf2-mediated signaling in response to OS led to the loss of quadriceps muscles [69]. In vitro, microgravity downregulated the transcription of mitochondrial uncoupling protein 2 (UCP2), which is a sensor and antidote for ROS production by mitochondria in skeletal muscle cells [74]. Targeting the Nrf2/Nox2 pathway has emerged as one of the ideas for treating muscle atrophy caused by microgravity.
3.2. Effects of OS on the Cardiovascular System in Microgravity
Microgravity causes adaptive changes in the cardiovascular system. For instance, it turned the heart into a rounder shape [75]. Studies have shown that total cardiac work is lower during spaceflight [76]. Microgravity also significantly affects the structure and function of vessels. As an illustration, microgravity increased the vessel wall thickness and the intima–media area in the basilar artery and decreased the wall thickness of the femoral artery, which might be consequences of the modulation of the content of focal adhesions (FAs) in the vessels by microgravity [77]. In vitro, microgravity caused structural and functional changes in endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) [78,79].
A majority of studies have shown that microgravity increases OS in the cardiovascular system, causing abnormalities of the myocardial and vascular structure and function (Table 2). Microgravity-induced OS had a negative impact on cardiovascular health [80,81]. In contrast, another study has shown that the cardiovascular system can adapt to microgravity without noticeable abnormalities [82]. Tahimic et al. found that cardiovascular oxidative damage was sex-dependent, and it was more pronounced in women [83].
Table 2.
Overview of the effects of OS on the cardiovascular system.
| Type of Tissues/Cells | Model | Study Result | Refs. |
|---|---|---|---|
| Ventricle | FLT | Contribute to cardiac dysfunction by altering the expression of genes regulating redox balance, cell cycle, and senescence | [81] |
| Hearts | HLU | Lead myocardial atrophy and dysfunction via increasing Rac1 activity | [84] |
| Heart tissues neonatal mouse cardiomyocytes |
HLU RCCS |
Promote myocardial abnormalities by facilitating p47phox phosphorylation via ERK1/2 and p38 pathways | [85] |
| Heart tissues | HLU | Induce myocardial dysfunction | [86] |
| EHTs | ISS | Induce mitochondrial dysfunction | [87] |
| HUVECs | FLT | Activate inflammatory responses, alter endothelial behavior, promote senesce | [88] |
| Cerebral and mesenteric VSMCs | HLU | Regulate cerebrovascular redox status and participate in vascular injury | [89] |
| Cerebral and mesenteric VSMCs | HLU | Result in cerebrovascular mitochondrial dysfunction | [90,91] |
| Brain HBMECs |
HLU 3D clinostat |
Induce BBB dysfunction via Rac1/Wave2/Arp3 signaling pathway | [92] |
| Brain | ISS | Indicate a disturbance of BBB integrity | [93] |
| Basilar and common carotid artery | HLU | Enhance maximal contractile response and impair endothelium-dependent relaxation | [94] |
| Basilar and common carotid artery | HLU | Enhance maximal contractile response and impair endothelium-dependent relaxation through an ANG II/AT1 receptor signaling pathway | [95] |
VSMCs: vascular smooth muscle cells; EHTs: engineered human heart tissues; HUVECs: human umbilical vein endothelial cells.
Microgravity-induced OS causes myocardial abnormalities. Microgravity activated NADPH oxidase, increased ROS content, and decreased MDA expression in the heart [84]. This phenomenon was mediated by the inhibition of p47phox phosphorylation on Ser345 via the ERK1/2 and p38 pathways [85,86]. Ser345 is an important step in the initiation of NADPH oxidase activation [96]. By using engineered human heart tissues (EHTs), Mair et al. sequenced RNA from the tissues after spaceflight and found that they exhibited mitochondrial dysfunction and impaired lipid oxidation, with increased ROS production. The same study also revealed a potential link between OS and mitochondrial dysfunction in microgravity environments through computer simulations, which corresponded to the RNA sequencing results [87].
Microgravity causes increased superoxide in blood vessels, resulting in vascular abnormalities. In human umbilical vein endothelial cells (HUVECs), spaceflight was closely associated with upregulated thioredoxin-interacting protein (TXNIP) and downregulated OXPHOS genes, which triggered mitochondrial dysfunction. Both mitochondrial dysfunction and TXNIP overexpression contribute to a pro-oxidant environment, leading to OS and promoting DNA damage and inflammation [88]. Moreover, simulated microgravity activated NADPH enzymes and increased ROS in the cerebral vasculature [89,90,91]. ROS in the cerebral vasculature activated matrix metalloproteinase-9 (MMP-9), which triggered the upregulation of aquaporin-4 (AQP4), impairing the integrity of the blood–brain barrier (BBB) and creating vasogenic brain edema [92,93,97]. Increased superoxide levels in the basilar and carotid arteries in simulated microgravity resulted in an enhanced maximal contractile response [94,98]. The increase in superoxide levels was regulated by the ANGII/AT1 receptor signaling pathway [95]. Interestingly, it appears that not all blood vessels are influenced by microgravity. Some studies have found that simulated microgravity has no effect on superoxide levels in the mesenteric arteries and mesenteric smooth muscle cells [89,90,91]. In summary, microgravity-induced OS causes myocardial abnormalities via ERK1/2 and p38 pathways, enhances the maximal contractile response of vessels, and promotes DNA damage of the vein endothelial cells in the cardiovascular system. However, some studies have also shown that microgravity does not cause oxidative stress in some blood vessels. The effects of OS on the cardiovascular system in microgravity need to be explored further.
3.3. Effects of OS on the Brain in Microgravity
Microgravity modifies the structure and function of the brain. It was shown to move the body fluids toward the head, with microstructural changes in cerebral white matter and the redistribution of cerebral spinal fluid (CSF), developing a hydrocephalus-like structure, which damages brain tissue and affects brain function [8]. Once astronauts return to the ground, BBB permeability increases, which might lead to symptoms such as optic disc edema and cognitive dysfunction [99]. Spaceflight also decreased the expression of crucial genes involved in dopamine (DA) synthesis and degradation in the brain, as well as the D1 receptor [100]. This finding significantly contributes to explaining the dyskinesia and motor incoordination observed in astronauts resulting from exposure to spaceflight conditions. In addition, Marotta et al. created a brain-like organ composed of cortical and DA neurons, which serves as a model for studying neurons affected by multiple sclerosis and Parkinson’s disease. Brain-like organs were cultured on the International Space Station (ISS) for one month. Organoids grown in microgravity were found to have higher levels of gene expression associated with maturation and lower levels of gene expression associated with proliferation [101]. This means that in microgravity, cells develop faster and replicate less.
Several studies have shown that microgravity causes OS in the brain (Table 3). Microgravity-induced OS primarily alters cortical and hippocampal function, resulting in a neurodegenerative-like condition. The enrichment of mitochondrial metabolic proteins in the hippocampus of rats in simulated microgravity revealed that mitochondrial electron transport, oxidative regulation, fatty acid metabolism, ATP metabolism, and responses to OS were downregulated [102]. Some studies also found that microgravity increases the levels of Nrf2, SOD, and peroxides in the hippocampus, leading to neuroinflammation and learning and memory deficits [103,104,105]. 4-Hydroxy-2-nonenal (4-HNE) is one of various aldehydes produced during lipid peroxidation. It reacts with proteins, DNA, and phospholipids, acting as an inducer and mediator of OS [106]. Microgravity modulated the increase in 4-HNE in the cortex and hippocampus by increasing Nox2 protein levels, leading to OS and damage to brain tissue [18,20]. However, by performing measurements in spaceflight mice within 2 days of splashing down, Mao et al. found that pathways related to cell death, repair, inflammation, and metabolic stress were significantly modified, but there were no significant differences in 4-HNE levels [107]. The different levels of 4-HNE in these studies were probably related to the differences in the models. Moreover, Thy-1 protein in the hypothalamus was upregulated in microgravity. Thy-1 increased ROS and induced OS [108,109]. Microgravity causes OS in the brain, but the effects and mechanisms of microgravity-induced OS in the brain are still little well studied.
Table 3.
Overview of the effects of OS on the brain.
| Type of Tissues/Cells | Model | Study Result | Refs. |
|---|---|---|---|
| Hippocampus | ISS | Increase in apoptosis | [93] |
| Hippocampus | HLU | Induce cognitive impairment by downregulation of the Sirt1/Nrf2 signaling pathway | [105] |
| Cortex; hippocampus | HLU | Cause learning and memory impairments | [104] |
| Hippocampus | HLU | Affect the function of hippocampus | [103] |
| Cortex | HLU | Induce endothelial damage and neurovascular remodeling | [18] |
| Cortex; hippocampus | HLU | Increase the likelihood of brain injury | [20] |
3.4. Effects of OS on the Immune System in Microgravity
Microgravity influences the proliferation, differentiation, activation, metabolism, and structure of immune cells, thereby impacting their normal functioning [110]. It was found that the total number of immune cells decreased but the number of neutrophils increased in microgravity, possibly because IL-8 induced the release of more neutrophils from the bone marrow [111]. Microgravity also affects the secretion of cytokines, including pro-inflammatory and anti-inflammatory cytokines. The release of pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6 was increased in microgravity [112]. The microgravity environment impacts the differentiation and metabolism of immune cells. Spaceflight and simulated microgravity significantly reduced hematopoietic progenitor cell (HPC) differentiation, decreased macrophage numbers and M1/M2 polarization, and led to metabolic reprogramming [113]. Microgravity downregulated MHC class II molecules and CD56 on the surface of DCs, inhibiting the activity of T cells [114]. Moreover, microgravity altered the migration and killing function of immune cells. In microgravity, the killing ability of natural killer (NK) cells was decreased [115]. The phagocytosis function of macrophages was also modified [116].
Currently, there are fewer studies related to the effects of OS on immune cells in microgravity. Blood from astronauts showed mild but persistent OS due to the increased production of superoxide and nitric oxide in granulocytes [117]. Moreover, it exhibited glutathione peroxidase 1 (GPX1) downregulation. GPX1 protects cells from oxidative damage and modulates the immune response [118]. The detection of CD4+ T cells from three astronauts showed features of persistent DNA damage response, including OS, inflammation, and telomere aberrations [119]. Additionally, Kaufmann et al. exposed polymorphonuclear leukocytes to parabolic flight and found that adenosine limited microgravity-induced OS by upregulating adenosine A2A receptor function. This anti-inflammatory signal was stronger than the signal in the normal physiological situation and may limit further cytotoxic damage [120]. In contrast, parabolic flight experiments with human leukemia Jurkat cells and monoblastic U937 cells revealed that the latter had 100% adapted after 5 min of being exposed to microgravity. OS-related genes were detected in human Jurkat cells that showed almost no response to microgravity [121]. Further studies on OS in immune cells in microgravity are required.
3.5. Effects of OS on Other Organisms in Microgravity
Microgravity also induces OS in the liver, skin, lung, intestine, and reproductive system. Microgravity caused a rise in ferroportin in the liver [122]. Increased ferroportin protein levels contributed to OS in liver disease pathology [123]. Another study also showed the upregulation of a set of genes associated with OS in the livers of mice from the ISS, which led to liver damage after spaceflight [124]. Microgravity increased the susceptibility of ECs to OS after exposure to lipopolysaccharide, upregulated Nrf2 target genes in skin fibroblasts, and affected skin functions [125,126]. Liu et al. discovered that the genes involved in OS, DNA repair, and fatty acid oxidation were activated in WI-38 human embryonic lung cells in a spaceflight experiment [127]. Consistently, Wang et al. identified the upregulation of receptor for advanced glycation endproducts (RAGE) and the downregulation of peroxiredoxin 1 (PRDX1) in the lung tissues of rats in simulated microgravity. These were associated with OS and played important roles in microgravity-induced lung injury [128]. The ileal epithelial cells of mice in simulated microgravity exhibited an increased level of OS, which led to impaired ecological niche function of intestinal stem cells (ISCs) and caused dysbiosis [129]. Moreover, microgravity induced OS in Hodgkin’s lymphoma and neuroblastoma [130,131]. Interestingly, Berardini et al. observed a significant increase in mitochondrial O2•− levels in TCam-2 spermatogonia in microgravity, and the expression of enzymes involved in redox homeostasis was also regulated to compensate for this. It was shown that these cells were able to trigger compensatory mechanisms that allowed them to overcome the regulation of microgravity [132]. In addition, microgravity had no significant effect on spongiosa [133]. Oxidative markers in saliva and serum were increased in a simulated microgravity environment, but there was no significant effect on periodontal parameters [134].
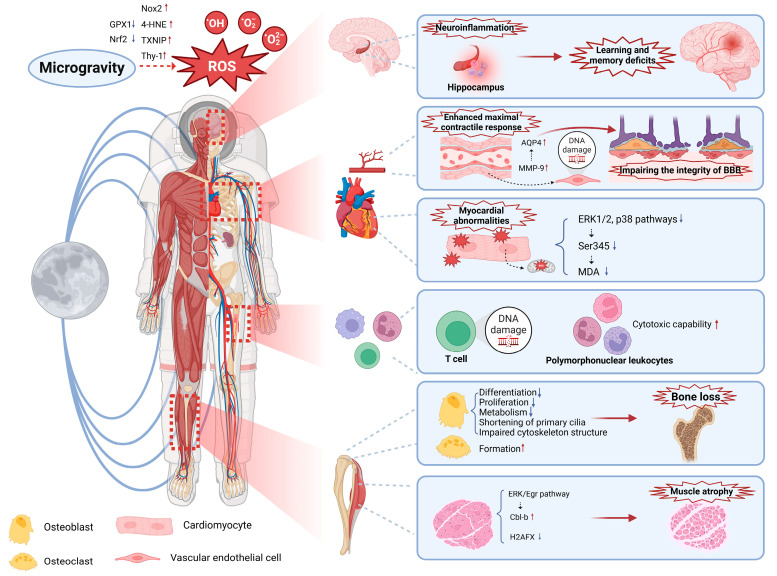
In summary, OS is enhanced in microgravity and affects multiple systems, such as the musculoskeletal, cardiovascular, nervous, and immune systems, and so on, causing damage to the organism (Figure 1).
Figure 1.
Effects of OS on the organism in microgravity. OS can cause dysfunction in the body, such as neuroinflammation, learning and memory deficits, myocardial and vascular abnormalities, impairment of the integrity of the BBB, oxidative damage to immune cells, bone loss, and skeletal muscle atrophy. BBB: blood–brain barrier.
4. Therapy for OS on the Ground
The main therapeutic options for OS are reducing ROS, inhibiting downstream signaling, increasing antioxidant enzymes and their substrates, and repairing damage caused by OS. ROS produced due to outside environmental factors could be reduced by modifying lifestyle, such as avoiding smoking, radiation, and environmental pollution [15]. In organisms, •OH is the key to oxidative damage. In vivo, the formation of •OH, which is highly oxidizing, was prevented by reducing the production of O2•− and H2O2 [25]. Fe3+Cyt c, acting as an antioxidant, also converts O2•− into molecular oxygen [135]. Molecular hydrogen (H2) reduces the production of O2•− by altering the direction of electron flow and neutralizing semiquinone radicals [136]. Moreover, environmental pollution led to the activation of NF-κB signaling in macrophages, which was directly inhibited by the addition of POD mimics. However, as H2O2 plays an important role in redox signaling and acts as a second messenger, its direct regulation led to abnormal signal transduction [137]. Phosphatidylcholine-specific phospholipase C (PC-PLC) was activated in this process. The inhibitor of PC-PLC, tricyclodecan-9-yl-xanthogenate (D609), is not an antioxidant but inhibited OS induced signal transduction [138]. The levels of antioxidant enzymes and their substrates were increased through exogenous supplementation or by activating antioxidant enzyme transcription factors. Dietary intake of vitamin C, vitamin E, and carotenoids enhanced the antioxidant capacity [139]. The expression of antioxidant enzymes like NQO1 and HO-1 was promoted through the activation of transcription factors, such as Nrf2, to neutralize oxygen free radicals and reduce oxidative damage [140,141]. The improvement in antioxidant enzyme activity, particularly that of quinone, increased the antioxidant capacity of cells [142]. Enzymes related to oxidative DNA damage repair, including FPG, AP endonucleases, and DNA polymerase, treated the damage caused by OS [34]. A considerable number of studies have been performed on treatments for OS on the ground, and the mechanism is clear.
5. Therapeutic Strategies for OS in Microgravity
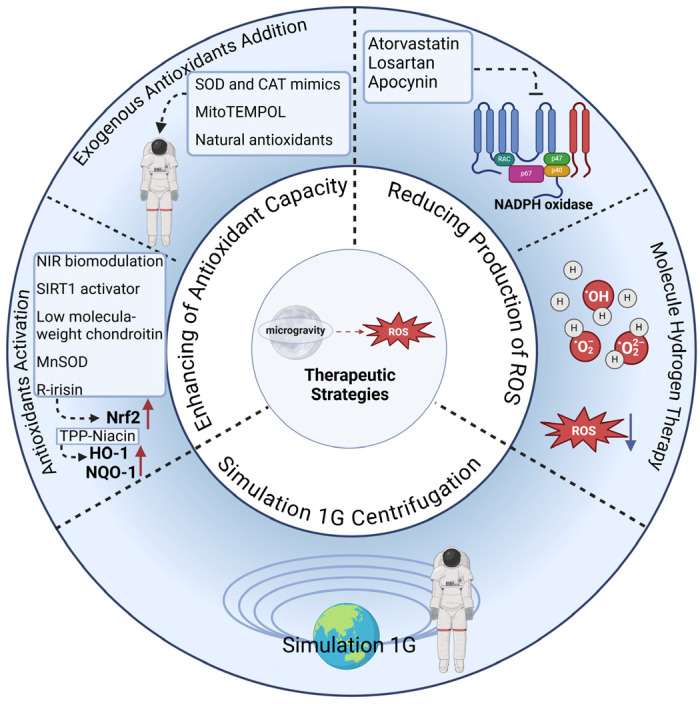
There are three strategies for treating microgravity-induced OS: reducing the production of ROS, enhancing the antioxidant capacity, and simulating the terrestrial environment through 1G centrifugation. The production of ROS was directly reduced by inhibiting oxidase activity or neutralizing peroxides. Atorvastatin, losartan, and apocynin mitigated myocardial and vascular dysfunction by inhibiting NADPH oxidase, thereby reducing superoxide production [84,86,89,91]. Sun et al. used molecular hydrogen therapy to protect bone in simulated microgravity. In vivo, mice were fed hydrogen water (HW), and in vitro, cells were incubated with hydrogen-rich medium (HRM). It was found that hydrogen molecules reduced bone loss by suppressing osteoblast differentiation, osteoclast production, and ROS [62]. There are two ways to improve antioxidant capacity: exogenous antioxidant addition and antioxidant activation induction in vivo. A mimetic of SOD and CAT (EUK-134) was effective in attenuating skeletal muscle atrophy in microgravity [143]. Mito-TEMPO, a mitochondria-targeted antioxidant, reduced NADPH oxidase activity by attenuating mitochondrial fission via the upregulation of MFN1/2 and the downregulation of DRP1/FIS1, thereby attenuating enhanced vasoconstriction in cerebral arterioles of rats in simulated microgravity [91,95,144]. A number of natural antioxidants have also shown considerable potential for the treatment of microgravity-induced OS. Salidroside suppressed ROS production by activating the Nrf2/HO-1 signaling pathway and reduced microgravity-induced OS, thereby playing a bone-protective role and preventing bone loss [58]. Curcumin protected against bone loss by inhibiting ROS formation induced by microgravity and by enhancing osteoblast differentiation [60]. Natural flavonoids also protected the osteogenic potential of osteoblasts by reducing OS in microgravity [64]. Moreover, near-infrared (NIR) biomodulation, the SIRT1 activator, low-molecular-weight chondroitin, the mitochondrial antioxidant enzyme MnSOD, and r-irisin decreased microgravity-induced OS and reduced tissue damage via the activation of the Nrf2/Nox2 pathway [19,59,68,105,145]. TPP-Niacin also downregulated microgravity-induced ROS formation in ARPE19 cells (adult retinal pigment epithelial cells) by promoting the expression of antioxidant-related genes such as HO-1 and NQO-1 [146]. Currently, there is a treatment program for microgravity: the simulation of the terrestrial environment through 1G centrifugation. Kurosawa et al. exposed mice to 1G centrifugation in a space station and found that this partially attenuated liver injury due to OS in microgravity [124]. Mao et al. demonstrated that performing 1G centrifugation on the ISS effectively reduced endothelial cell damage and enhanced cellular organization and function compared with the microgravity group [107]. These studies provide new ideas for treating OS caused by microgravity (Table 4, Figure 2). However, there are few therapeutic strategies for microgravity-induced OS, and the mechanisms are unclear. More studies exploring the mechanisms and development of therapeutic options for microgravity-induced OS are needed.
Table 4.
Overview of the therapeutic strategies for OS in microgravity.
| Type of Tissues/Cells | Therapeutic Strategy | Treatment | Study Result | Refs. |
|---|---|---|---|---|
| Heart | Direct reduction of ROS | Atorvastatin | Inhibit Rac1 activation to attenuate myocardial atrophy | [84] |
| Heart | Losartan | Preserve cardiomyocyte size and prevent myocardial dysfunction by blocking Ser345 and NADPH oxidase activation | [86] | |
| Cerebral VSMCs | Apocynin | Regulate cerebrovascular redox status with NADPH oxidase inhibition; promote recovery of mitochondrial function | [89,91] | |
| Femur; lumbar vertebra MC3T3-E1; RAW264.7 cells |
HW & HRM | Alleviate microgravity-induced bone loss | [62] | |
| Cerebral VSMCs | Enhancement of antioxidant capacity | MitoTEMPOL | Promote recovery of mitochondrial function | [91] |
| Basilar and common carotid artery | Losartan | Through ANGII/AT1 receptor signaling pathway | [95] | |
| Femur MC3T3-E1 cells |
Salidroside | Mitigate bone loss induced by stimulating the Nrf2/HO-1 pathway | [58] | |
| Tibiae; Femur MC3T3-E1; RAW264.7 cells |
Ccurcumin | Preserve bone structure and mechanical strength by upregulating VDR expression in femurs | [60] | |
| Rat calvarial osteoblasts | Natural flavonoid moslosooflavone | Mitigate loss of osteogenic potential of osteoblasts by protecting primary cilium | [64] | |
| Hippocampus | PBM | Mitigate cognitive impairment through the activation of the Sirt1/Nrf2 signaling pathway, reduction in OS | [105] | |
| Gastrocnemius and soleus | SRT2104 | Preserve mitochondrial function to prevent skeletal muscle atrophy | [68] | |
| Femur | LMWCSs | Protect against the bone loss related to reduce OS | [59] | |
| Human osteoblasts | R-irisin | Prevent apoptotic death | [145] | |
| ARPE19 cells | TPP-Niacin | Reduce ROS elevation by promoting the expression of antioxidant-related genes | [146] | |
| Liver | Simulation of the terrestrial environment | 1G centrifugation | Mitigate liver damage | [124] |
| ocular | Provide protection against changes | [107] |
VDR: Vitamin D Receptor; PBM: Photobiomodulation; HW: Hydrogen Water; HRM: Hydrogen-Rich Medium; LMWCSs: Low-Molecular-Weight Chondroitin Sulfates.
Figure 2.
Therapeutic strategies for OS in microgravity. There are currently three strategies for treating microgravity-induced OS: the direct reduction of ROS by inhibiting oxidase activity or neutralizing peroxides, the enhancement of antioxidant capacity via exogenous antioxidant addition and inducing antioxidant activation induction, and exposure to 1G centrifugation. NIR: near-infrared.
6. Conclusions
This review documents current studies related to microgravity-induced OS as a significant factor in spaceflight causing dysfunction in the body as follows: bone loss and skeletal muscle atrophy, myocardial and vascular abnormalities, learning and memory deficits, oxidative damage to immune cells, liver damage, skin dysfunction, and lung damage. In the future, studying the mechanisms of microgravity-induced OS will not only contribute to our understanding of the effects on human health in space but also support the development of new therapeutic strategies. Some studies have also shown that cells rapidly adapt to microgravity without experiencing OS. The mechanisms of adaptation to microgravity remain to be further explored. Currently, the effects of microgravity-induced OS on organs are not yet well understood. To summarize, the mechanisms underlying microgravity-induced OS hold significant potential for further exploration, which would contribute to subsequent targeted therapy for microgravity-induced OS.
The exploration of therapeutic strategies offers potential solutions to reduce OS in microgravity. At present, there are three strategies for treating microgravity-induced OS: the direct reduction of ROS, the enhancement of antioxidant capacity, and exposure to 1G centrifugation. Overall, therapies for treating OS in microgravity remain underexplored, and further exploration will not only be essential for astronaut health during spaceflight but also provide new insights for the treatment of OS-related diseases on the ground.
In conclusion, this review presents a preliminary introduction and summary of the topic of microgravity-induced OS and therapeutic strategies pertaining to this condition. The study of microgravity-induced OS presents a promising avenue for future research. As space exploration progresses, research in this area will become vital to protecting astronaut health and advancing the field of space medicine. Subsequent research should focus on the mechanisms by which microgravity induces OS, the mechanisms by which microgravity-induced OS affects organs, and potential treatments for mitigating OS in space.
Abbreviations
The following abbreviations are used in this manuscript:
| TME | Tumor microenvironment. |
| ROS | Reactive oxygen species. |
| OS | Oxidative stress. |
| SAS | Space adaptation syndrome. |
| SMS | Space motion sickness. |
| ICP | Intracranial pressure. |
| SANS | Spaceflight-associated neuro-ocular syndrome. |
| NADPH | Nicotinamide adenine dinucleotide phosphate. |
| NOXs | Nicotinamide adenine dinucleotide phosphate oxidases. |
| Nrf2 | Nuclear transcription factor red lineage 2-related factor 2. |
| AREs | Antioxidant response elements. |
| HLU | Hindlimb unloading. |
| O2•− | Superoxide anion. |
| H2O2 | Hydrogen peroxide. |
| •OH | Hydroxyl radicals. |
| 8-oxoG | 8-dihydroguanine. |
| OXPHOS | Oxidative phosphorylation. |
| SOD | Superoxide dismutase. |
| CAT | Catalase. |
| POD | Peroxidase. |
| APX | Ascorbate peroxidase. |
| vitamin C | Ascorbic acid. |
| vitamin E | Alpha-tocopherol. |
| GSH | Glutathione. |
| FPG | DNA glycosylase. |
| OGG1 | 8-oxoguanine DNA glycosylase. |
| DCs | Dendritic cells. |
| TRPV4 | Transient receptor potential vanilloid 4. |
| LDH | Lactate dehydrogenase. |
| Cbl-b | Casitas B-lineage lymphoma-b. |
| D609 | Tricyclodecan-9-yl-xanthogenate. |
| Egr | ERK1/2 early growth response protein. |
| H2AFX | H2A histone family member X. |
| SIRT1 | Sirtuin 1. |
| UCP2 | Uncoupling protein 2. |
| FAs | Focal adhesions. |
| ECs | Endothelial cells. |
| VSMCs | Vascular smooth muscle cells. |
| EHTs | Engineered human heart tissues. |
| HUVECs | Human umbilical vein endothelial cells. |
| TXNIP | Thioredoxin-interacting protein. |
| MMP-9 | Matrix metalloproteinase-9. |
| AQP4 | Aquaporin-4. |
| BBB | Blood–brain barrier. |
| CSF | Cerebral spinal fluid. |
| DA | Dopamine. |
| ISS | International Space Station. |
| MDA | Malondialdehyde. |
| 4-HNE | 4-Hydroxy-2-nonenal. |
| HPCs | Hematopoietic progenitor cells. |
| NK | Natural killer. |
| GPX1 | Glutathione peroxidase 1. |
| RAGE | Receptor for advanced glycation endproducts. |
| PRDX1 | Peroxiredoxin 1. |
| ISCs | Intestinal stem cells. |
| H2 | Molecular hydrogen. |
| PC-PLC | Phosphatidylcholine-specific phospholipase C. |
| HW | Hydrogen water. |
| HRM | Hydrogen-rich medium. |
| NIR | Near-infrared. |
Author Contributions
Writing—original draft preparation, X.Z. and H.Z.; writing—review and editing, X.Z. and J.Z.; supervision and funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China (81972689), the Natural Science Foundation of Beijing (7232102), the Scientific and Technological Innovation Major Base of Guangxi (No. 2022-36-Z05), and the Guangxi Science and Technology Major Program (No. AA24263028).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Afshinnekoo E., Scott R.T., MacKay M.J., Pariset E., Cekanaviciute E., Barker R., Gilroy S., Hassane D., Smith S.M., Zwart S.R., et al. Fundamental biological features of spaceflight: Advancing the field to enable deep-space exploration. Cell. 2020;183:1162–1184. doi: 10.1016/j.cell.2020.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran Q.D., Tran V., Toh L.S., Williams P.M., Tran N.N., Hessel V. Space medicines for space health. ACS Med. Chem. Lett. 2022;13:1231–1247. doi: 10.1021/acsmedchemlett.1c00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russomano T., Da Rosa M., Dos Santos M.A. Space motion sickness: A common neurovestibular dysfunction in microgravity. Neurol. India. 2019;67((Suppl. S2)):S214–S218. doi: 10.4103/0028-3886.259127. [DOI] [PubMed] [Google Scholar]
- 4.Lee A.G., Tarver W.J., Mader T.H., Gibson C.R., Hart S.F., Otto C.A. Neuro-ophthalmology of space flight. J. Neuro-Ophthalmol. 2016;36:85–91. doi: 10.1097/WNO.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 5.Kadipasaoglu C.M., Lee V.A., Ong J., Lee A.G. The optic nerve in spaceflight: Novel concepts in the pathogenesis of optic disc edema in microgravity. Curr. Opin. Neurol. 2024;38:87–95. doi: 10.1097/WCO.0000000000001334. [DOI] [PubMed] [Google Scholar]
- 6.Zwart S.R., Gibson C.R., Gregory J.F., Mader T.H., Stover P.J., Zeisel S.H., Smith S.M. Astronaut ophthalmic syndrome. FASEB J. 2017;31:3746–3756. doi: 10.1096/fj.201700294. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.K., Koppelmans V., Riascos R.F., Hasan K.M., Pasternak O., Mulavara A.P., Bloomberg J.J., Seidler R.D. Spaceflight-associated brain white matter microstructural changes and intracranial fluid redistribution. JAMA Neurol. 2019;76:412–419. doi: 10.1001/jamaneurol.2018.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Ombergen A., Jillings S., Jeurissen B., Tomilovskaya E., Rumshiskaya A., Litvinova L., Nosikova I., Pechenkova E., Rukavishnikov I., Manko O., et al. Brain ventricular volume changes induced by long-duration spaceflight. Proc. Natl. Acad. Sci. USA. 2019;116:10531–10536. doi: 10.1073/pnas.1820354116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trappe T.A., Tesch P., Alkner B., Trappe S. Microgravity-induced skeletal muscle atrophy in women and men: Implications for long-duration spaceflights to the Moon and Mars. J. Appl. Physiol. 2023;135:1115–1119. doi: 10.1152/japplphysiol.00412.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Man J., Graham T., Squires-Donelly G., Laslett A.L. The effects of microgravity on bone structure and function. npj Microgravity. 2022;8:9. doi: 10.1038/s41526-022-00194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi J., Wang Y., He J., Li P., Jin R., Wang K., Xu X., Hao J., Zhang Y., Liu H., et al. Intestinal microbiota contributes to colonic epithelial changes in simulated microgravity mouse model. FASEB J. 2017;31:3695–3709. doi: 10.1096/fj.201700034R. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui R., Akbar N., Khan N.A. Gut microbiome and human health under the space environment. J. Appl. Microbiol. 2021;130:14–24. doi: 10.1111/jam.14789. [DOI] [PubMed] [Google Scholar]
- 13.Buchheim J.-I., Matzel S., Rykova M., Vassilieva G., Ponomarev S., Nichiporuk I., Hörl M., Moser D., Biere K., Feuerecker M., et al. Stress related shift toward inflammaging in cosmonauts after long-duration space flight. Front. Physiol. 2019;10:85. doi: 10.3389/fphys.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang T.T., Walther I., Li C.-F., Boonyaratanakornkit J., Galleri G., Meloni M.A., Pippia P., Cogoli A., Hughes-Fulford M. The Rel/Nf-κB pathway and transcription of immediate early genes in T cell activation are inhibited by microgravity. J. Leukoc. Biol. 2012;92:1133–1145. doi: 10.1189/jlb.0312157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahoo B.M., Banik B.K., Borah P., Jain A. Reactive oxygen species (ROS): Key components in cancer therapies. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2022;22:215–222. doi: 10.2174/1871520621666210608095512. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K., Okumura H., Guo R., Naruse K. Effect of oxidative stress on cardiovascular system in response to gravity. Int. J. Mol. Sci. 2017;18:1426. doi: 10.3390/ijms18071426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan S., Pei W., Huang H., Zhou G., Hu W. Additive effects of simulated microgravity and ionizing radiation in cell death, induction of ROS and expression of RAC2 in human bronchial epithelial cells. npj Microgravity. 2020;6:34. doi: 10.1038/s41526-020-00123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao X.W., Nishiyama N.C., Campbell-Beachler M., Gifford P., Haynes K.E., Gridley D.S., Pecaut M.J. Role of NADPH oxidase as a mediator of oxidative damage in low-dose irradiated and hindlimb-unloaded mice. Radiat. Res. 2017;188:392–399. doi: 10.1667/RR14754.1. [DOI] [PubMed] [Google Scholar]
- 19.Lawler J.M., Hord J.M., Ryan P., Holly D., Janini Gomes M., Rodriguez D., Guzzoni V., Garcia-Villatoro E., Green C., Lee Y., et al. Nox2 inhibition regulates stress response and mitigates skeletal muscle fiber atrophy during simulated microgravity. Int. J. Mol. Sci. 2021;22:3252. doi: 10.3390/ijms22063252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao X.W., Nishiyama N.C., Pecaut M.J., Campbell-Beachler M., Gifford P., Haynes K.E., Becronis C., Gridley D.S. Simulated microgravity and low-dose/low-dose-rate radiation induces oxidative damage in the mouse brain. Radiat. Res. 2016;185:647–657. doi: 10.1667/RR14267.1. [DOI] [PubMed] [Google Scholar]
- 21.Cao S., Wang Y., Zhang Y., Ren J., Fan B., Deng Y., Yin W. Naringenin can Inhibit the Pyroptosis of Osteoblasts by Activating the Nrf2/HO-1 Signaling Pathway and Alleviate the Differentiation Disorder of Osteoblasts Caused by Microgravity. J. Agric. Food Chem. 2024;72:25586–25600. doi: 10.1021/acs.jafc.4c05370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulasov A.V., Rosenkranz A.A., Georgiev G.P., Sobolev A.S. Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci. 2022;291:120111. doi: 10.1016/j.lfs.2021.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sies H., Cadenas E. Oxidative stress: Damage to intact cells and organs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985;311:617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 24.Rahal A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S., Dhama K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forman H.J., Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shields H.J., Traa A., Van Raamsdonk J.M. Beneficial and detrimental effects of reactive oxygen species on lifespan: A comprehensive review of comparative and experimental studies. Front. Cell Dev. Biol. 2021;9:628157. doi: 10.3389/fcell.2021.628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shadel G.S., Horvath T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin H., Wang D., Wang X. A novel module regulating ROS in NLR-mediated immunity. Trends Plant Sci. 2023;28:512–514. doi: 10.1016/j.tplants.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Rizwan S., ReddySekhar P., MalikAsrar B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halliwell B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024;25:13–33. doi: 10.1038/s41580-023-00645-4. [DOI] [PubMed] [Google Scholar]
- 32.Park E., Chung S. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. doi: 10.1038/s41419-019-2064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sies H., Belousov V.V., Chandel N.S., Davies M.J., Jones D.P., Mann G.E., Murphy M.P., Yamamoto M., Winterbourn C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022;23:499–515. doi: 10.1038/s41580-022-00456-z. [DOI] [PubMed] [Google Scholar]
- 34.Boiteux S., Coste F., Castaing B. Repair of 8-oxo-7, 8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases. Free Radic. Biol. Med. 2017;107:179–201. doi: 10.1016/j.freeradbiomed.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 35.Li X., Zhuang X., Qiao T. Role of ferroptosis in the process of acute radiation-induced lung injury in mice. Biochem. Biophys. Res. Commun. 2019;519:240–245. doi: 10.1016/j.bbrc.2019.08.165. [DOI] [PubMed] [Google Scholar]
- 36.Ge W., Zhao K., Wang X., Li H., Yu M., He M., Xue X., Zhu Y., Zhang C., Cheng Y., et al. iASPP is an antioxidative factor and drives cancer growth and drug resistance by competing with Nrf2 for Keap1 binding. Cancer Cell. 2017;32:561–573.e6. doi: 10.1016/j.ccell.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Wu X., Zhang H., Qi W., Zhang Y., Li J., Li Z., Lin Y., Bai X., Liu X., Chen X., et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018;9:171. doi: 10.1038/s41419-017-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An Y., Zhang H., Wang C., Jiao F., Xu H., Wang X., Luan W., Ma F., Ni L., Tang X., et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019;33:12515. doi: 10.1096/fj.201802805RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu L., Zhang K., Sandoval H., Yamamoto S., Jaiswal M., Sanz E., Li Z., Hui J., Graham B.H., Quintana A., et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160:177–190. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elbedwehy A.M., Wu J., Na H.-K., Baek A., Jung H., Kwon I.H., Lee S.W., Kim J.H., Lee T.G. ROS-responsive charge reversal mesoporous silica nanoparticles as promising drug delivery system for neovascular retinal diseases. J. Control. Release. 2024;373:224–239. doi: 10.1016/j.jconrel.2024.07.022. [DOI] [PubMed] [Google Scholar]
- 41.Zhu C., Shen S., Zhang S., Huang M., Zhang L., Chen X. Autophagy in bone remodeling: A regulator of oxidative stress. Front. Endocrinol. 2022;13:898634. doi: 10.3389/fendo.2022.898634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X., Fan D., Cao X., Ye Q., Wang Q., Zhang M., Xiao C. The role of reactive oxygen species in the rheumatoid arthritis-associated synovial microenvironment. Antioxidants. 2022;11:1153. doi: 10.3390/antiox11061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X., Gao J., Wu C., Wang C., Zhang R., He J., Xia Z.J., Joshi N., Karp J.M., Kuai R., et al. Precise modulation and use of reactive oxygen species for immunotherapy. Sci. Adv. 2024;10:eadl0479. doi: 10.1126/sciadv.adl0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glorieux C., Liu S., Trachootham D., Huang P. Targeting ROS in cancer: Rationale and strategies. Nat. Rev. Drug Discov. 2024;23:583–606. doi: 10.1038/s41573-024-00979-4. [DOI] [PubMed] [Google Scholar]
- 45.Liu S., Huang B., Cao J., Wang Y., Xiao H., Zhu Y., Zhang H. ROS fine-tunes the function and fate of immune cells. Int. Immunopharmacol. 2023;119:110069. doi: 10.1016/j.intimp.2023.110069. [DOI] [PubMed] [Google Scholar]
- 46.Matsushita M., Freigang S., Schneider C., Conrad M., Bornkamm G.W., Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 2015;212:555–568. doi: 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cortés-Sánchez J.L., Callant J., Krüger M., Sahana J., Kraus A., Baselet B., Infanger M., Baatout S., Grimm D. Cancer studies under space conditions: Finding answers abroad. Biomedicines. 2021;10:25. doi: 10.3390/biomedicines10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miglietta S., Cristiano L., Espinola M.S.B., Masiello M.G., Micara G., Battaglione E., Linari A., Palmerini M.G., Familiari G., Aragona C. Effects of simulated microgravity in vitro on human metaphase II oocytes: An electron microscopy-based study. Cells. 2023;12:1346. doi: 10.3390/cells12101346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Globus R.K., Morey-Holton E. Hindlimb unloading: Rodent analog for microgravity. J. Appl. Physiol. 2016;120:1196–1206. doi: 10.1152/japplphysiol.00997.2015. [DOI] [PubMed] [Google Scholar]
- 50.Hargens A.R., Vico L. Long-duration bed rest as an analog to microgravity. J. Appl. Physiol. 2016;120:891–903. doi: 10.1152/japplphysiol.00935.2015. [DOI] [PubMed] [Google Scholar]
- 51.Tomilovskaya E., Shigueva T., Sayenko D., Rukavishnikov I., Kozlovskaya I. Dry immersion as a ground-based model of microgravity physiological effects. Front. Physiol. 2019;10:284. doi: 10.3389/fphys.2019.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishimura Y. Technology using simulated microgravity. Regen. Ther. 2023;24:318–323. doi: 10.1016/j.reth.2023.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robling A.G., Castillo A.B., Turner C.H. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 54.Grimm D., Grosse J., Wehland M., Mann V., Reseland J.E., Sundaresan A., Corydon T.J. The impact of microgravity on bone in humans. Bone. 2016;87:44–56. doi: 10.1016/j.bone.2015.12.057. [DOI] [PubMed] [Google Scholar]
- 55.Dakkumadugula A., Pankaj L., Alqahtani A.S., Ullah R., Ercisli S., Murugan R. Space nutrition and the biochemical changes caused in Astronauts Health due to space flight: A review. Food Chem. X. 2023;20:100875. doi: 10.1016/j.fochx.2023.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bayle D., Coudy-Gandilhon C., Gueugneau M., Castiglioni S., Zocchi M., Maj-Zurawska M., Palinska-Saadi A., Mazur A., Béchet D., Maier J.A., et al. Magnesium deficiency alters expression of genes critical for muscle magnesium homeostasis and physiology in mice. Nutrients. 2021;13:2169. doi: 10.3390/nu13072169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinha S., Elbaz-Alon Y., Avinoam O. Ca2+ as a coordinator of skeletal muscle differentiation, fusion and contraction. FEBS J. 2022;289:6531–6542. doi: 10.1111/febs.16552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang N., Zuo Z., Meng T., Liu Y., Zheng X., Ma Y. Salidroside alleviates simulated microgravity-induced bone loss by activating the Nrf2/HO-1 pathway. J. Orthop. Surg. Res. 2024;19:531. doi: 10.1186/s13018-024-05030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lan R., Li Y., Zhao X., Shen R., Wang R., Mao R., Guo S. Low-Molecular-Weight Chondroitin Sulfates Alleviate Simulated Microgravity-Induced Oxidative Stress and Bone Loss in Mice. Curr. Issues Mol. Biol. 2023;45:4214–4227. doi: 10.3390/cimb45050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xin M., Yang Y., Zhang D., Wang J., Chen S., Zhou D. Attenuation of hind-limb suspension-induced bone loss by curcumin is associated with reduced oxidative stress and increased vitamin D receptor expression. Osteoporos. Int. 2015;26:2665–2676. doi: 10.1007/s00198-015-3153-7. [DOI] [PubMed] [Google Scholar]
- 61.Michaletti A., Gioia M., Tarantino U., Zolla L. Effects of microgravity on osteoblast mitochondria: A proteomic and metabolomics profile. Sci. Rep. 2017;7:15376. doi: 10.1038/s41598-017-15612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun Y., Shuang F., Chen D., Zhou R. Treatment of hydrogen molecule abates oxidative stress and alleviates bone loss induced by modeled microgravity in rats. Osteoporos. Int. 2013;24:969–978. doi: 10.1007/s00198-012-2028-4. [DOI] [PubMed] [Google Scholar]
- 63.Morabito C., Guarnieri S., Cucina A., Bizzarri M., Mariggiò M.A. Antioxidant strategy to prevent simulated microgravity-induced effects on bone osteoblasts. Int. J. Mol. Sci. 2020;21:3638. doi: 10.3390/ijms21103638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miao L.W., Liu T.Z., Sun Y.H., Cai N., Xuan Y.Y., Wei Z., Cui B.B., Jing L.L., Ma H.P., Xian C.J., et al. Simulated microgravity-induced oxidative stress and loss of osteogenic potential of osteoblasts can be prevented by protection of primary cilia. J. Cell. Physiol. 2023;238:2692–2709. doi: 10.1002/jcp.31127. [DOI] [PubMed] [Google Scholar]
- 65.Reich K.A., Chen Y.-W., Thompson P.D., Hoffman E.P., Clarkson P.M. Forty-eight hours of unloading and 24 h of reloading lead to changes in global gene expression patterns related to ubiquitination and oxidative stress in humans. J. Appl. Physiol. 2010;109:1404–1415. doi: 10.1152/japplphysiol.00444.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uchida T., Sakashita Y., Kitahata K., Yamashita Y., Tomida C., Kimori Y., Komatsu A., Hirasaka K., Ohno A., Nakao R., et al. Reactive oxygen species upregulate expression of muscle atrophy-associated ubiquitin ligase Cbl-b in rat L6 skeletal muscle cells. Am. J. Physiol.-Cell Physiol. 2018;314:C721–C731. doi: 10.1152/ajpcell.00184.2017. [DOI] [PubMed] [Google Scholar]
- 67.Genchi G.G., Degl’Innocenti A., Salgarella A.R., Pezzini I., Marino A., Menciassi A., Piccirillo S., Balsamo M., Ciofani G. Modulation of gene expression in rat muscle cells following treatment with nanoceria in different gravity regimes. Nanomedicine. 2018;13:2821–2833. doi: 10.2217/nnm-2018-0316. [DOI] [PubMed] [Google Scholar]
- 68.Wesolowski L.T., Simons J.L., Semanchik P.L., Othman M.A., Kim J.-H., Lawler J.M., Kamal K.Y., White-Springer S.H. The impact of SRT2104 on skeletal muscle mitochondrial function, redox biology, and loss of muscle mass in hindlimb unloaded rats. Int. J. Mol. Sci. 2023;24:11135. doi: 10.3390/ijms241311135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chakraborty N., Waning D.L., Gautam A., Hoke A., Sowe B., Youssef D., Butler S., Savaglio M., Childress P.J., Kumar R., et al. Gene-metabolite network linked to inhibited bioenergetics in association with spaceflight-induced loss of male mouse quadriceps muscle. J. Bone Miner. Res. 2020;35:2049–2057. doi: 10.1002/jbmr.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Onishi Y., Hirasaka K., Ishihara I., Oarada M., Goto J., Ogawa T., Suzue N., Nakano S., Furochi H., Ishidoh K., et al. Identification of mono-ubiquitinated LDH-A in skeletal muscle cells exposed to oxidative stress. Biochem. Biophys. Res. Commun. 2005;336:799–806. doi: 10.1016/j.bbrc.2005.08.175. [DOI] [PubMed] [Google Scholar]
- 71.Georg T., Thomas B., Pauline M., Angèle C., Guillemette G.-K., Stéphane B., Fabrice B. Proteome-wide Adaptations of Mouse Skeletal Muscles during a Full Month in Space. J. Proteome Res. 2017;16:2623–2638. doi: 10.1021/acs.jproteome.7b00201. [DOI] [PubMed] [Google Scholar]
- 72.Kubben N., Zhang W., Wang L., Voss T.C., Yang J., Qu J., Liu G.-H., Misteli T. Repression of the antioxidant NRF2 pathway in premature aging. Cell. 2016;165:1361–1374. doi: 10.1016/j.cell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weyemi U., Paul B.D., Snowman A.M., Jailwala P., Nussenzweig A., Bonner W.M., Snyder S.H. Histone H2AX deficiency causes neurobehavioral deficits and impaired redox homeostasis. Nat. Commun. 2018;9:1526. doi: 10.1038/s41467-018-03948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Genchi G.G., Degl’Innocenti A., Martinelli C., Battaglini M., De Pasquale D., Prato M., Marras S., Pugliese G., Drago F., Mariani A., et al. Cerium oxide nanoparticle administration to skeletal muscle cells under different gravity and radiation conditions. ACS Appl. Mater. Interfaces. 2021;13:40200–40213. doi: 10.1021/acsami.1c14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iskovitz I., Kassemi M., Thomas J.D. Impact of weightlessness on cardiac shape and left ventricular stress/strain distributions. J. Biomech. Eng. 2013;135:121008. doi: 10.1115/1.4025464. [DOI] [PubMed] [Google Scholar]
- 76.Shibata S., Wakeham D.J., Thomas J.D., Abdullah S.M., Platts S., Bungo M.W., Levine B.D. Cardiac effects of long-duration space flight. J. Am. Coll. Cardiol. 2023;82:674–684. doi: 10.1016/j.jacc.2023.05.058. [DOI] [PubMed] [Google Scholar]
- 77.Jiang M., Lyu Q., Bai Y.-G., Liu H., Yang J., Cheng J.-H., Zheng M., Ma J. Focal adhesions are involved in simulated-microgravity-induced basilar and femoral arterial remodelling in rats. Can. J. Physiol. Pharmacol. 2018;96:772–782. doi: 10.1139/cjpp-2017-0665. [DOI] [PubMed] [Google Scholar]
- 78.Xu K., Wang X., Bai H., Wu G., Zhang W., Zhou J., Zhang P., Zhang X., Peng B., Voelcker N.H., et al. A biosensory μvessel-gravity device for advancing vascular analysis in space medicine. Biosens. Bioelectron. 2025;268:116923. doi: 10.1016/j.bios.2024.116923. [DOI] [PubMed] [Google Scholar]
- 79.Grenon S.M., Jeanne M., Aguado-Zuniga J., Conte M.S., Hughes-Fulford M. Effects of gravitational mechanical unloading in endothelial cells: Association between caveolins, inflammation and adhesion molecules. Sci. Rep. 2013;3:1494. doi: 10.1038/srep01494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seawright J.W., Samman Y., Sridharan V., Mao X.W., Cao M., Singh P., Melnyk S., Koturbash I., Nelson G.A., Hauer-Jensen M., et al. Effects of low-dose rate γ-irradiation combined with simulated microgravity on markers of oxidative stress, DNA methylation potential, and remodeling in the mouse heart. PLoS ONE. 2017;12:e0180594. doi: 10.1371/journal.pone.0180594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar A., Tahimic C.G., Almeida E.A., Globus R.K. Spaceflight modulates the expression of key oxidative stress and cell cycle related genes in heart. Int. J. Mol. Sci. 2021;22:9088. doi: 10.3390/ijms22169088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veliz A.L., Mamoun L., Hughes L., Vega R., Holmes B., Monteon A., Bray J., Pecaut M.J., Kearns-Jonker M. Transcriptomic effects on the mouse heart following 30 days on the international space station. Biomolecules. 2023;13:371. doi: 10.3390/biom13020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tahimic C.G., Steczina S., Sebastian A., Hum N.R., Abegaz M., Terada M., Cimini M., Goukassian D.A., Schreurs A.-S., Hoban-Higgins T.M., et al. Simulated Microgravity Alters Gene Regulation Linked to Immunity and Cardiovascular Disease. Genes. 2024;15:975. doi: 10.3390/genes15080975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li H., Cao T., Ding W., Liang L., Fan G.-C., Qu L., Peng T. Pharmacological inhibition of Rac1 attenuates myocardial abnormalities in tail-suspended mice. J. Cardiovasc. Transl. Res. 2022;15:805–815. doi: 10.1007/s12265-021-10197-7. [DOI] [PubMed] [Google Scholar]
- 85.Liang L., Li H., Cao T., Qu L., Zhang L., Fan G.-C., Greer P.A., Li J., Jones D.L., Peng T., et al. Calpain activation mediates microgravity-induced myocardial abnormalities in mice via p38 and ERK1/2 MAPK pathways. J. Biol. Chem. 2020;295:16840–16851. doi: 10.1074/jbc.RA119.011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang L., Yuan W., Qu L., Li H., Zhang L., Fan G.-C., Peng T. Administration of losartan preserves cardiomyocyte size and prevents myocardial dysfunction in tail-suspended mice by inhibiting p47 phox phosphorylation, NADPH oxidase activation and MuRF1 expression. J. Transl. Med. 2019;17:279. doi: 10.1186/s12967-019-2021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mair D.B., Tsui J.H., Higashi T., Koenig P., Dong Z., Chen J.F., Meir J.U., Smith A.S., Lee P.H., Ahn E.H., et al. Spaceflight-induced contractile and mitochondrial dysfunction in an automated heart-on-a-chip platform. Proc. Natl. Acad. Sci. USA. 2024;121:e2404644121. doi: 10.1073/pnas.2404644121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Versari S., Longinotti G., Barenghi L., Maier J.A.M., Bradamante S. The challenging environment on board the International Space Station affects endothelial cell function by triggering oxidative stress through thioredoxin interacting protein overexpression: The ESA-SPHINX experiment. FASEB J. 2013;27:4466–4475. doi: 10.1096/fj.13-229195. [DOI] [PubMed] [Google Scholar]
- 89.Liang P., Ran H.H., Zhang Y., Yu Z., Fan Y.Y., Li P., Zhang R., Feng C. NADPH oxidase accounts for changes in cerebrovascular redox status in hindlimb unweighting rats. Biomed. Environ. Sci. 2015;28:799–807. doi: 10.3967/bes2015.111. [DOI] [PubMed] [Google Scholar]
- 90.Zhang R., Ran H.-H., Peng L., Xu F., Sun J.-F., Zhang L.-N., Fan Y.-Y., Peng L., Cui G. Mitochondrial regulation of NADPH oxidase in hindlimb unweighting rat cerebral arteries. PLoS ONE. 2014;9:e95916. doi: 10.1371/journal.pone.0095916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang R., Ran H.H., Cai L.L., Zhu L., Sun J.F., Peng L., Liu X.J., Zhang L.N., Fang Z., Fan Y.Y., et al. Simulated microgravity-induced mitochondrial dysfunction in rat cerebral arteries. FASEB J. 2014;28:2715–2724. doi: 10.1096/fj.13-245654. [DOI] [PubMed] [Google Scholar]
- 92.Yan R., Liu H., Lv F., Deng Y., Li Y. Rac1/Wave2/Arp3 pathway mediates rat blood-brain barrier dysfunction under simulated microgravity based on proteomics strategy. Int. J. Mol. Sci. 2021;22:5165. doi: 10.3390/ijms22105165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mao X.W., Nishiyama N.C., Byrum S.D., Stanbouly S., Jones T., Holley J., Sridharan V., Boerma M., Tackett A.J., Willey J.S., et al. Spaceflight induces oxidative damage to blood-brain barrier integrity in a mouse model. FASEB J. 2020;34:15516. doi: 10.1096/fj.202001754R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang R., Ran H.-H., Ma J., Bai Y.-G., Lin L.-J. NAD (P) H oxidase inhibiting with apocynin improved vascular reactivity in tail-suspended hindlimb unweighting rat. J. Physiol. Biochem. 2012;68:99–105. doi: 10.1007/s13105-011-0123-1. [DOI] [PubMed] [Google Scholar]
- 95.Zhang R., Bai Y.-G., Lin L.-J., Bao J.-X., Zhang Y.-Y., Tang H., Cheng J.-H., Jia G.-L., Ren X.-L., Ma J., et al. Blockade of AT1 receptor partially restores vasoreactivity, NOS expression, and superoxide levels in cerebral and carotid arteries of hindlimb unweighting rats. J. Appl. Physiol. 2009;106:251–258. doi: 10.1152/japplphysiol.01278.2007. [DOI] [PubMed] [Google Scholar]
- 96.El-Benna J., Dang P.M.-C., Périanin A. Peptide-based inhibitors of the phagocyte NADPH oxidase. Biochem. Pharmacol. 2010;80:778–785. doi: 10.1016/j.bcp.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 97.Katsu M., Niizuma K., Yoshioka H., Okami N., Sakata H., Chan P.H. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood–brain barrier dysfunction in vivo. J. Cereb. Blood Flow Metab. 2010;30:1939–1950. doi: 10.1038/jcbfm.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang R., Ran H.-H., Gao Y.-L., Ma J., Huang Y., Bai Y.-G., Lin L.-J. Differential vascular cell adhesion molecule-1 expression and superoxide production in simulated microgravity rat vasculature. EXCLI J. 2010;9:195. [PMC free article] [PubMed] [Google Scholar]
- 99.Seidler R.D., Mao X.W., Tays G.D., Wang T., Zu Eulenburg P. Effects of spaceflight on the brain. Lancet Neurol. 2024;23:826–835. doi: 10.1016/S1474-4422(24)00224-2. [DOI] [PubMed] [Google Scholar]
- 100.Kharlamova A., Proshchina A., Gulimova V., Krivova Y., Soldatov P., Saveliev S. Cerebellar morphology and behavioural correlations of the vestibular function alterations in weightlessness. Neurosci. Biobehav. Rev. 2021;126:314–328. doi: 10.1016/j.neubiorev.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 101.Marotta D., Ijaz L., Barbar L., Nijsure M., Stein J., Pirjanian N., Kruglikov I., Clements T., Stoudemire J., Grisanti P., et al. Effects of microgravity on human iPSC-derived neural organoids on the International Space Station. Stem Cells Transl. Med. 2024;13:1186–1197. doi: 10.1093/stcltm/szae070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y., Javed I., Liu Y., Lu S., Peng G., Zhang Y., Qing H., Deng Y. Effect of prolonged simulated microgravity on metabolic proteins in rat hippocampus: Steps toward safe space travel. J. Proteome Res. 2016;15:29–37. doi: 10.1021/acs.jproteome.5b00777. [DOI] [PubMed] [Google Scholar]
- 103.Wang T., Chen H., Lv K., Ji G., Liang F., Zhang Y., Wang Y., Liu X., Cao H., Kan G., et al. Activation of HIF-1α and its downstream targets in rat hippocampus after long-term simulated microgravity exposure. Biochem. Biophys. Res. Commun. 2017;485:591–597. doi: 10.1016/j.bbrc.2016.12.078. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Y., Wang Q., Chen H., Liu X., Lv K., Wang T., Wang Y., Ji G., Cao H., Kan G., et al. Involvement of cholinergic dysfunction and oxidative damage in the effects of simulated weightlessness on learning and memory in rats. BioMed Res. Int. 2018;2018:2547532. doi: 10.1155/2018/2547532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kazmi S., Farajdokht F., Meynaghizadeh-Zargar R., Sadigh-Eteghad S., Pasokh A., Farzipour M., Farazi N., Hamblin M.R., Mahmoudi J. Transcranial photobiomodulation mitigates learning and memory impairments induced by hindlimb unloading in a mouse model of microgravity exposure by suppression of oxidative stress and neuroinflammation signaling pathways. Brain Res. 2023;1821:148583. doi: 10.1016/j.brainres.2023.148583. [DOI] [PubMed] [Google Scholar]
- 106.Li Y., Zhao T., Li J., Xia M., Li Y., Wang X., Liu C., Zheng T., Chen R., Kan D., et al. Oxidative stress and 4-hydroxy-2-nonenal (4-HNE): Implications in the pathogenesis and treatment of aging-related diseases. J. Immunol. Res. 2022;2022:2233906. doi: 10.1155/2022/2233906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mao X.W., Byrum S., Nishiyama N.C., Pecaut M.J., Sridharan V., Boerma M., Tackett A.J., Shiba D., Shirakawa M., Takahashi S., et al. Impact of spaceflight and artificial gravity on the mouse retina: Biochemical and proteomic analysis. Int. J. Mol. Sci. 2018;19:2546. doi: 10.3390/ijms19092546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen J., Li H.-Y., Wang D., Guo X.-Z. Delphinidin protects β2m−/Thy1+ bone marrow-derived hepatocyte stem cells against TGF-β1-induced oxidative stress and apoptosis through the PI3K/Akt pathway in vitro. Chem.-Biol. Interact. 2019;297:109–118. doi: 10.1016/j.cbi.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 109.Iqbal J., Li W., Hasan M., Juan Li Y., Ullah K., Yun W., Awan U., Qing H., Deng Y. Distortion of homeostatic signaling proteins by simulated microgravity in rat hypothalamus: A16 O/18 O-labeled comparative integrated proteomic approach. Proteomics. 2014;14:262–273. doi: 10.1002/pmic.201300337. [DOI] [PubMed] [Google Scholar]
- 110.Lv H., Yang H., Jiang C., Shi J., Chen R.-A., Huang Q., Shao D. Microgravity and immune cells. J. R. Soc. Interface. 2023;20:20220869. doi: 10.1098/rsif.2022.0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loos T., Opdenakker G., Van Damme J., Proost P. Citrullination of CXCL8 increases this chemokine’s ability to mobilize neutrophils into the blood circulation. Haematologica. 2009;94:1346. doi: 10.3324/haematol.2009.006973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ludtka C., Silberman J., Moore E., Allen J.B. Macrophages in microgravity: The impact of space on immune cells. npj Microgravity. 2021;7:13. doi: 10.1038/s41526-021-00141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shi L., Tian H., Wang P., Li L., Zhang Z., Zhang J., Zhao Y. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell. Mol. Immunol. 2021;18:1489–1502. doi: 10.1038/s41423-019-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Savary C.A., Grazziutti M.L., Przepiorka D., Tomasovic S.P., McINTYRE B.W., Woodside D.G., Pellis N.R., Pierson D.L., Rex J.H. Characteristics of human dendritic cells generated in a microgravity analog culture system. Vitr. Cell. Dev. Biol.-Anim. 2001;37:216–222. doi: 10.1007/BF02577532. [DOI] [PubMed] [Google Scholar]
- 115.Bigley A.B., Agha N.H., Baker F.L., Spielmann G., Kunz H.E., Mylabathula P.L., Rooney B.V., Laughlin M.S., Mehta S.K., Pierson D.L., et al. NK cell function is impaired during long-duration spaceflight. J. Appl. Physiol. 2019;126:842–853. doi: 10.1152/japplphysiol.00761.2018. [DOI] [PubMed] [Google Scholar]
- 116.Wang S., Zhang N., Di J., Zhao W., Shi G., Xie R., Hu B., Yang H. Analysis of the effects of magnetic levitation to simulate microgravity environment on the Arp2/3 complex pathway in macrophage. J. Biol. Phys. 2021;47:323–335. doi: 10.1007/s10867-021-09581-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Luca C., Deeva I., Mariani S., Maiani G., Stancato A., Korkina L. Monitoring antioxidant defenses and free radical production in space-flight, aviation and railway engine operators, for the prevention and treatment of oxidative stress, immunological impairment, and pre-mature cell aging. Toxicol. Ind. Health. 2009;25:259–267. doi: 10.1177/0748233709103032. [DOI] [PubMed] [Google Scholar]
- 118.Barrila J., Ott C.M., LeBlanc C., Mehta S.K., Crabbé A., Stafford P., Pierson D.L., Nickerson C.A. Spaceflight modulates gene expression in the whole blood of astronauts. npj Microgravity. 2016;2:16039. doi: 10.1038/npjmgrav.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luxton J.J., McKenna M.J., Taylor L.E., George K.A., Zwart S.R., Crucian B.E., Drel V.R., Garrett-Bakelman F.E., Mackay M.J., Butler D., et al. Temporal telomere and DNA damage responses in the space radiation environment. Cell Rep. 2020;33:108435. doi: 10.1016/j.celrep.2020.108435. [DOI] [PubMed] [Google Scholar]
- 120.Kaufmann I., Feuerecker M., Salam A., Schelling G., Thiel M., Choukèr A. Adenosine A2A receptor modulates the oxidative stress response of primed polymorphonuclear leukocytes after parabolic flight. Hum. Immunol. 2011;72:547–552. doi: 10.1016/j.humimm.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 121.Tauber S., Christoffel S., Thiel C.S., Ullrich O. Transcriptional homeostasis of oxidative stress-related pathways in altered gravity. Int. J. Mol. Sci. 2018;19:2814. doi: 10.3390/ijms19092814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cavey T., Pierre N., Nay K., Allain C., Ropert M., Loréal O., Derbré F. Simulated microgravity decreases circulating iron in rats: Role of inflammation-induced hepcidin upregulation. Exp. Physiol. 2017;102:291–298. doi: 10.1113/EP086188. [DOI] [PubMed] [Google Scholar]
- 123.McDonald C.J., Ostini L., Wallace D.F., John A.N., Watters D.J., Subramaniam V.N. Iron loading and oxidative stress in the Atm−/− mouse liver. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011;300:G554–G560. doi: 10.1152/ajpgi.00486.2010. [DOI] [PubMed] [Google Scholar]
- 124.Kurosawa R., Sugimoto R., Imai H., Atsuji K., Yamada K., Kawano Y., Ohtsu I., Suzuki K. Impact of spaceflight and artificial gravity on sulfur metabolism in mouse liver: Sulfur metabolomic and transcriptomic analysis. Sci. Rep. 2021;11:21786. doi: 10.1038/s41598-021-01129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beck M., Moreels M., Quintens R., Abou-El-Ardat K., El-Saghire H., Tabury K., Michaux A., Janssen A., Neefs M., Van Oostveldt P., et al. Chronic exposure to simulated space conditions predominantly affects cytoskeleton remodeling and oxidative stress response in mouse fetal fibroblasts. Int. J. Mol. Med. 2014;34:606–615. doi: 10.3892/ijmm.2014.1785. [DOI] [PubMed] [Google Scholar]
- 126.Chakraborty N., Gautam A., Muhie S., Miller S.-A., Jett M., Hammamieh R. An integrated omics analysis: Impact of microgravity on host response to lipopolysaccharide in vitro. BMC Genom. 2014;15:659. doi: 10.1186/1471-2164-15-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Y., Wang E. Transcriptional analysis of normal human fibroblast responses to microgravity stress. Genom. Proteom. Bioinform. 2008;6:29–41. doi: 10.1016/S1672-0229(08)60018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J., Liu C., Li T., Wang Y., Wang D. Proteomic analysis of pulmonary tissue in tail-suspended rats under simulated weightlessness. J. Proteom. 2012;75:5244–5253. doi: 10.1016/j.jprot.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 129.Zong B., Wang J., Wang K., Hao J., Han J.-Y., Jin R., Ge Q. Effects of Ginsenoside Rb1 on the Crosstalk between Intestinal Stem Cells and Microbiota in a Simulated Weightlessness Mouse Model. Int. J. Mol. Sci. 2024;25:8769. doi: 10.3390/ijms25168769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qu L., Chen H., Liu X., Bi L., Xiong J., Mao Z., Li Y. Protective effects of flavonoids against oxidative stress induced by simulated microgravity in SH-SY5Y cells. Neurochem. Res. 2010;35:1445–1454. doi: 10.1007/s11064-010-0205-4. [DOI] [PubMed] [Google Scholar]
- 131.Jeong A.J., Kim Y.J., Lim M.H., Lee H., Noh K., Kim B.-H., Chung J.W., Cho C.-H., Kim S., Ye S.-K., et al. Microgravity induces autophagy via mitochondrial dysfunction in human Hodgkin’s lymphoma cells. Sci. Rep. 2018;8:14646. doi: 10.1038/s41598-018-32965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berardini M., Gesualdi L., Morabito C., Ferranti F., Reale A., Zampieri M., Karpach K., Tinari A., Bertuccini L., Guarnieri S., et al. Simulated Microgravity Exposure Induces Antioxidant Barrier Deregulation and Mitochondria Enlargement in TCam-2 Cell Spheroids. Cells. 2023;12:2106. doi: 10.3390/cells12162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Andrade M.R., Azeez T.A., Montgomery M.M., Caldwell J.T., Park H., Kwok A.T., Borg A.M., Narayanan S.A., Willey J.S., Delp M.D., et al. Neurovascular dysfunction associated with erectile dysfunction persists after long-term recovery from simulations of weightlessness and deep space irradiation. FASEB J. 2023;37:e23246. doi: 10.1096/fj.202300506RR. [DOI] [PubMed] [Google Scholar]
- 134.Rai B., Kaur J., Catalina M., Anand S., Jacobs R., Teughels W. Effect of simulated microgravity on salivary and serum oxidants, antioxidants, and periodontal status. J. Periodontol. 2011;82:1478–1482. doi: 10.1902/jop.2011.100711. [DOI] [PubMed] [Google Scholar]
- 135.Velayutham M., Hemann C., Zweier J.L. Removal of H2O2 and generation of superoxide radical: Role of cytochrome c and NADH. Free. Radic. Biol. Med. 2011;51:160–170. doi: 10.1016/j.freeradbiomed.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ishihara G., Kawamoto K., Komori N., Ishibashi T. Molecular hydrogen suppresses superoxide generation in the mitochondrial complex I and reduced mitochondrial membrane potential. Biochem. Biophys. Res. Commun. 2020;522:965–970. doi: 10.1016/j.bbrc.2019.11.135. [DOI] [PubMed] [Google Scholar]
- 137.Forman H.J., Ursini F., Maiorino M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 2014;73:2–9. doi: 10.1016/j.yjmcc.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Human L.-S. A Phosphatidylcholine-Specific Phospholipase. J. Immunol. 1999;162:3005–3012. [PubMed] [Google Scholar]
- 139.Mirmiran P., Hosseini-Esfahani F., Esfandiar Z., Hosseinpour-Niazi S., Azizi F. Associations between dietary antioxidant intakes and cardiovascular disease. Sci. Rep. 2022;12:1504. doi: 10.1038/s41598-022-05632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yu J., Zhong B., Zhao L., Hou Y., Ai N., Lu J.-J., Ge W., Chen X. Fighting drug-resistant lung cancer by induction of NAD (P) H: Quinone oxidoreductase 1 (NQO1)-mediated ferroptosis. Drug Resist. Updates. 2023;70:100977. doi: 10.1016/j.drup.2023.100977. [DOI] [PubMed] [Google Scholar]
- 141.Duan C., Wang H., Jiao D., Geng Y., Wu Q., Yan H., Li C. Curcumin restrains oxidative stress of after intracerebral hemorrhage in rat by activating the Nrf2/HO-1 pathway. Front. Pharmacol. 2022;13:889226. doi: 10.3389/fphar.2022.889226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao L., Zheng L. A Review on Bioactive Anthraquinone and Derivatives as the Regulators for ROS. Molecules. 2023;28:8139. doi: 10.3390/molecules28248139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kuczmarski J.M., Hord J.M., Lee Y., Guzzoni V., Rodriguez D., Lawler M.S., Garcia-Villatoro E.L., Holly D., Ryan P., Falcon K., et al. Effect of Eukarion-134 on Akt–mTOR signalling in the rat soleus during 7 days of mechanical unloading. Exp. Physiol. 2018;103:545–558. doi: 10.1113/EP086649. [DOI] [PubMed] [Google Scholar]
- 144.Liu Z.F., Wang H.M., Jiang M., Lin W., Le Jian L., Zhao Y.Z., Shao J.J., Zhou J.J., Xie M.J., Xin L., et al. Mitochondrial oxidative stress enhances vasoconstriction by altering calcium homeostasis in cerebrovascular smooth muscle cells under simulated microgravity. Biomed. Environ. Sci. 2021;34:203–212. doi: 10.3967/bes2021.001. [DOI] [PubMed] [Google Scholar]
- 145.Cariati I., Bonanni R., Rinaldi A.M., Marini M., Iundusi R., Gasbarra E., Tancredi V., Tarantino U. Recombinant irisin prevents cell death and mineralization defects induced by random positioning machine exposure in primary cultures of human osteoblasts: A promising strategy for the osteoporosis treatment. Front. Physiol. 2023;14:1107933. doi: 10.3389/fphys.2023.1107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nguyen H.P., Shin S., Shin K.-J., Tran P.H., Park H., De Tran Q., No M.-H., Sun J.S., Kim K.W., Kwak H.-B., et al. Protective effect of TPP-Niacin on microgravity-induced oxidative stress and mitochondrial dysfunction of retinal epithelial cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2023;1870:119384. doi: 10.1016/j.bbamcr.2022.119384. [DOI] [PubMed] [Google Scholar]