Abstract
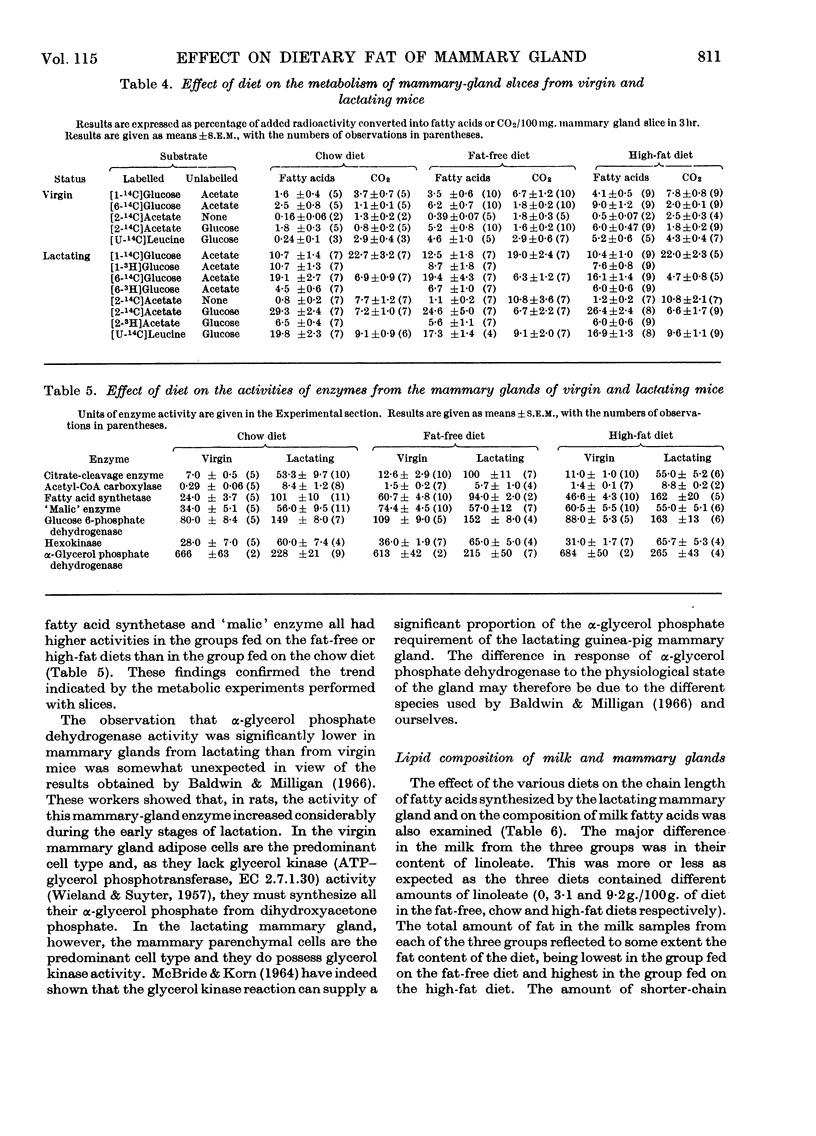
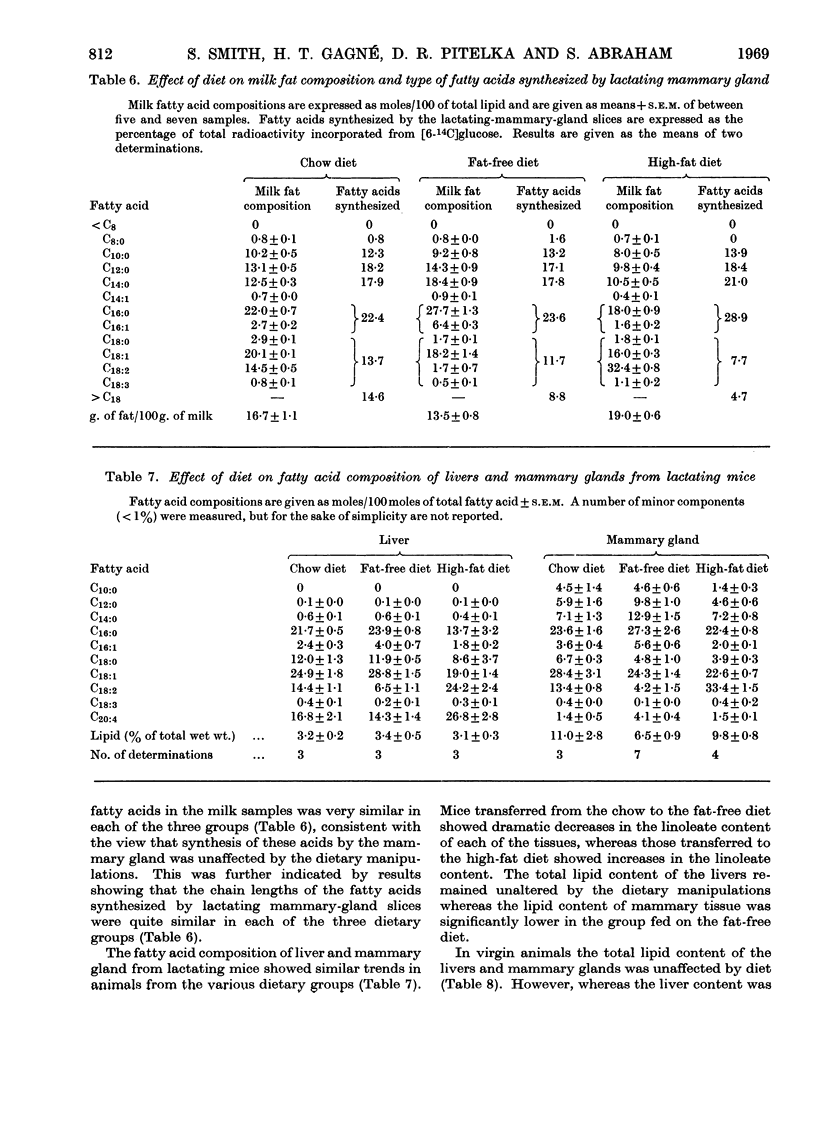
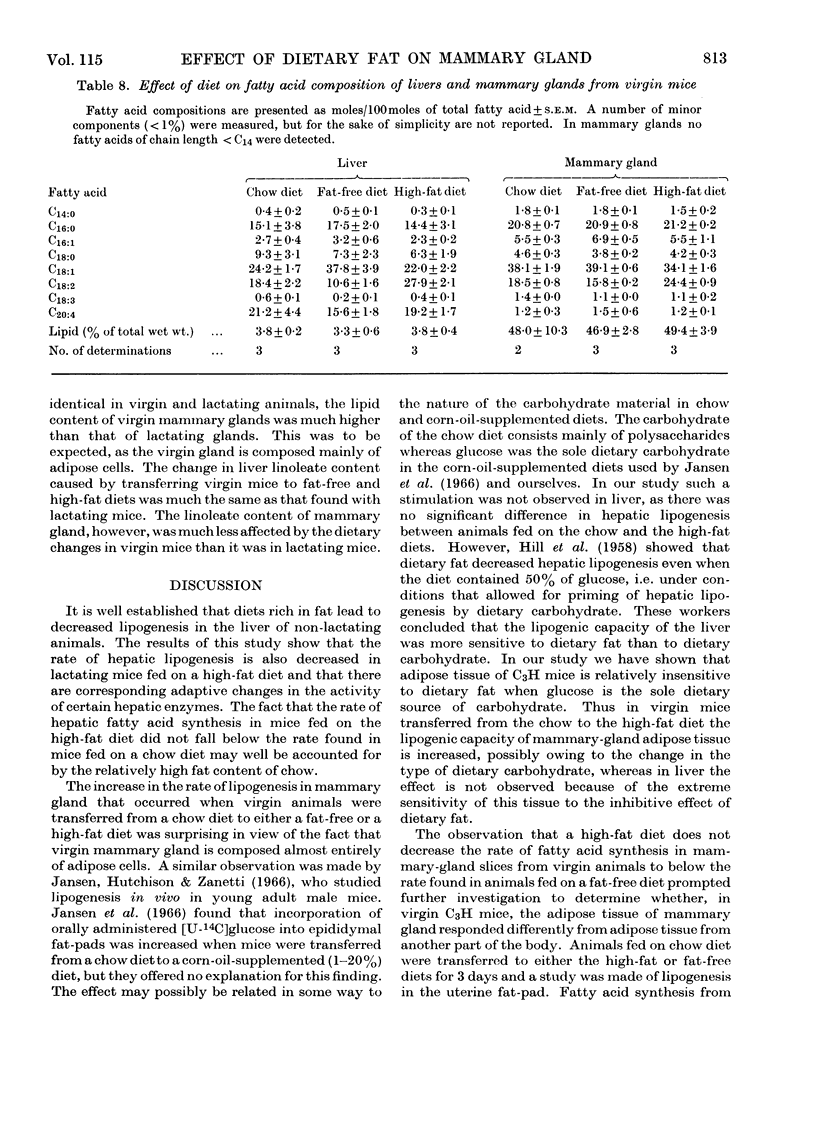
1. Virgin and lactating C3H mice maintained on laboratory chow were transferred to a high-fat (15% corn oil) or a fat-free diet 3 days before being killed. 2. The linoleate content of liver, mammary gland and milk was decreased in lactating mice given the fat-free diet but was increased in those fed on the high-fat diet. Changes in linoleate content and mammary gland followed a similar but much less marked trend in virgin animals. 3. Hepatic fatty acid synthesis in lactating and virgin mice fed on the fat-free diet was higher than in corresponding animals fed on either the chow or the high-fat diet. The lipogenic capacity of livers from mice fed on either the chow or the high-fat diet was greater in lactating than in virgin animals. These changes in hepatic lipogenic capacity were accompanied by alterations in the specific activities of certain enzymes involved in fat synthesis. 4. Mammary gland from virgin and lactating animals showed no such adaptation to dietary fat. Results indicate that fatty acid synthesis in neither mammary-gland parenchymal cells nor mammary-gland adipose cells can be influenced by dietary fat in the same way as in the hepatocyte.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., KOPELOVICH L., KERKOF P. R., CHAIKOFF I. L. METABOLIC CHARACTERISTICS OF PREPARATIONS OF ISOLATED SHEEP THYROID GLAND CELLS. I. ACTIVITY LEVELS OF ENZYMES CONCERNED WITH GLYCOLYSIS AND THE TRICARBOXYLIC ACID CYCLE. Endocrinology. 1965 Feb;76:178–190. doi: 10.1210/endo-76-2-178. [DOI] [PubMed] [Google Scholar]
- ABRAHAM S., MADSEN J., CHAIKOFF I. L. THE INFLUENCE OF GLUCOSE ON AMINO ACID CARBON INCORPORATION INTO PROTEINS, FATTY ACIDS, AND CARBON DIOXIDE BY LACTATING RAT MAMMARY GLAND SLICES. J Biol Chem. 1964 Mar;239:855–864. [PubMed] [Google Scholar]
- ABRAHAM S., MATTHES K. J., CHAIKOFF I. L. Factors involved in synthesis of fatty acids from acetate by a soluble fraction obtained from lactating rat mammary gland. Biochim Biophys Acta. 1961 May 13;49:268–285. doi: 10.1016/0006-3002(61)90127-5. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L., Milligan L. P. Enzymatic changes associated with the initiation and maintenance of lactation in the rat. J Biol Chem. 1966 May 10;241(9):2058–2066. [PubMed] [Google Scholar]
- Bartley J. C., Abraham S., Chaikoff I. L. Activity patterns of several enzymes of liver, adipose tissue, and mammary gland of virgin, pregnant, and lactating mice. Proc Soc Exp Biol Med. 1966 Dec;123(3):670–675. doi: 10.3181/00379727-123-31573. [DOI] [PubMed] [Google Scholar]
- Coniglio J. G., Culp F. B. The effect of fasting on fatty acid synthesis in cell-free preparations of rat mammary gland. Biochim Biophys Acta. 1965 Oct 4;106(2):419–421. doi: 10.1016/0005-2760(65)90052-4. [DOI] [PubMed] [Google Scholar]
- DANNENBURG W. N., BURT R. L., LEAKE N. H. LIPID COMPOSITION AND SYNTHESIS IN RAT LIVER DURING PREGNANCY AND THE PUERPERIUM. Proc Soc Exp Biol Med. 1964 Feb;115:504–508. doi: 10.3181/00379727-115-28952. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Foster D. W., Srere P. A. Citrate cleavage enzyme and fatty acid synthesis. J Biol Chem. 1968 Apr 25;243(8):1926–1930. [PubMed] [Google Scholar]
- Goodridge A. G. Conversion of[U-14C]glucose into carbon dioxide, glycogen, cholesterol and fatty acids in liver slices from embryonic and growing chicks. Biochem J. 1968 Jul;108(4):655–661. doi: 10.1042/bj1080655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSBERGER F. X., MILSTEIN S. W. Dietary effect on lipogenesis in adipose tissue. J Biol Chem. 1955 May;214(1):483–488. [PubMed] [Google Scholar]
- HILL R., BAKER N., CHAIKOFF I. L. Altered metabolic patterns induced in the normal rat by feeding an adequate diet containing fructose as sole carbohydrate. J Biol Chem. 1954 Aug;209(2):705–716. [PubMed] [Google Scholar]
- HILL R., LINAZASORO J. M., CHEVALLIER F., CHAIKOFF I. L. Regulation of hepatic lipogenesis: the influence of dietary fats. J Biol Chem. 1958 Aug;233(2):305–310. [PubMed] [Google Scholar]
- Jansen G. R., Hutchon C. F., Zanetti M. E. Studies on lipogenesis in vivo. Effect of dietary fat or starvation on conversion of [14]glucose into fat ad turnover of newly synthsized fat. Biochem J. 1966 May;99(2):323–332. doi: 10.1042/bj0990323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G. R., Zanetti M. E., Hutchison C. F. Studies on lipogenesis in vivo: Comparison of cholesterol and fatty acid synthesis in rats and mice. Biochem J. 1967 Mar;102(3):864–869. doi: 10.1042/bj1020864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. W. CITRATE CLEAVAGE AND ACETATE ACTIVATION IN LIVERS OF NORMAL AND DIABETIC RATS. Biochim Biophys Acta. 1964 Aug 5;84:490–492. doi: 10.1016/0926-6542(64)90021-6. [DOI] [PubMed] [Google Scholar]
- Kornacker M. S., Ball E. G. Citrate cleavage in adipose tissue. Proc Natl Acad Sci U S A. 1965 Sep;54(3):899–904. doi: 10.1073/pnas.54.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille G. A., Hanson R. W. Adaptive changes in enzyme activity and metabolic pathways in adipose tissue from meal-fed rats. J Lipid Res. 1966 Jan;7(1):46–55. [PubMed] [Google Scholar]
- McBride O. W., Korn E. D. Acceptors of fatty acid for glyceride synthesis in guinea pig mammary gland. J Lipid Res. 1964 Jul;5(3):448–452. [PubMed] [Google Scholar]
- McLEAN P. Carbohydrate metabolism of mammary tissue. I. Pathways of glucose catabolism in the mammary gland. Biochim Biophys Acta. 1958 Nov;30(2):303–315. doi: 10.1016/0006-3002(58)90055-6. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Peraino C., Pries N., Kennan A. L. Glucose repression and induction of enzyme synthesis in rat liver. Adv Enzyme Regul. 1964;2:237–247. doi: 10.1016/s0065-2571(64)80016-9. [DOI] [PubMed] [Google Scholar]
- SRERE P. A. The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem. 1959 Oct;234:2544–2547. [PubMed] [Google Scholar]
- Smith S., Easter D. J., Dils R. Fatty acid biosynthesis. 3. Intracellular site of enzymes in lactating-rabbit mammary gland. Biochim Biophys Acta. 1966 Dec 7;125(3):445–455. [PubMed] [Google Scholar]
- WHITNEY J. E., ROBERTS S. Influence of previous diet on hepatic glycogenesis and lipogenesis. Am J Physiol. 1955 May;181(2):446–450. doi: 10.1152/ajplegacy.1955.181.2.446. [DOI] [PubMed] [Google Scholar]
- WIELAND O., SUYTER M. Glycerokinase; Isolierung und Eigenschaften des Enzyms. Biochem Z. 1957;329(4):320–331. [PubMed] [Google Scholar]
- WILLMER J. S. The influence of adrenalectomy upon the activity of the hexosemonophosphate shunt in the livers and mammary glands of lactating rats. Can J Biochem Physiol. 1960 Nov;38:1265–1273. [PubMed] [Google Scholar]
- Wiese H. F., Coon E., Yamanaka W., Barber S., Johnson P. Lipid composition of the vascular system during infancy, childhood, and young adulthood. J Lipid Res. 1967 Jul;8(4):312–320. [PubMed] [Google Scholar]


