Abstract
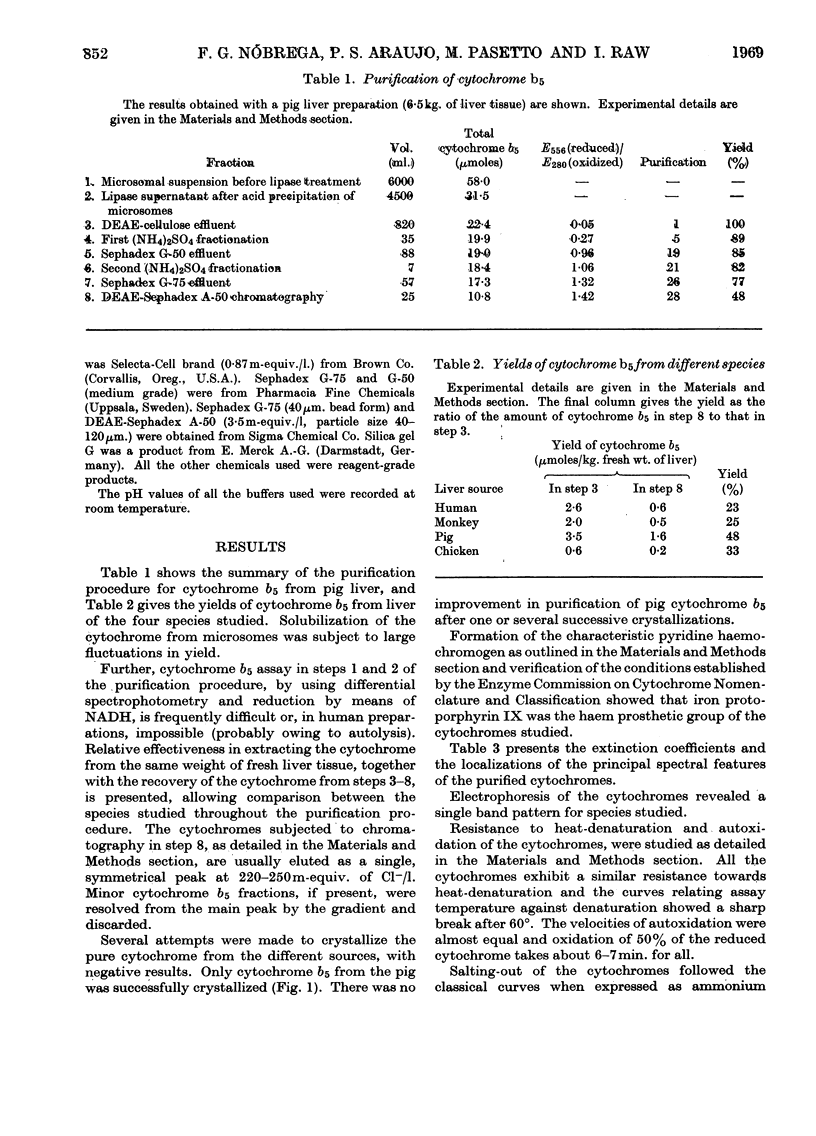
1. Cytochrome b5 was released from liver microsomes of man, monkey, pig and chicken by incubation with a crude lipase preparation. 2. By using DEAE-cellulose chromatography, ammonium sulphate fractionation, Sephadex-gel filtration and a final gradient elution on DEAE-Sephadex A-50, cytochromes b5 were obtained from the four species studied, all possessing similar spectral properties. 3. Stokes radii of the cytochromes were measured by gel filtration. 4. N-Terminal amino acids for the different cytochromes were serine for man and monkey, alanine for pig and glycine for chicken. 5. Amino acid analyses of the cytochromes are presented. 6. Peptide `fingerprint' patterns of tryptic digests of the different cytochromes are discussed and clearly show increasing similarity for more closely related species.
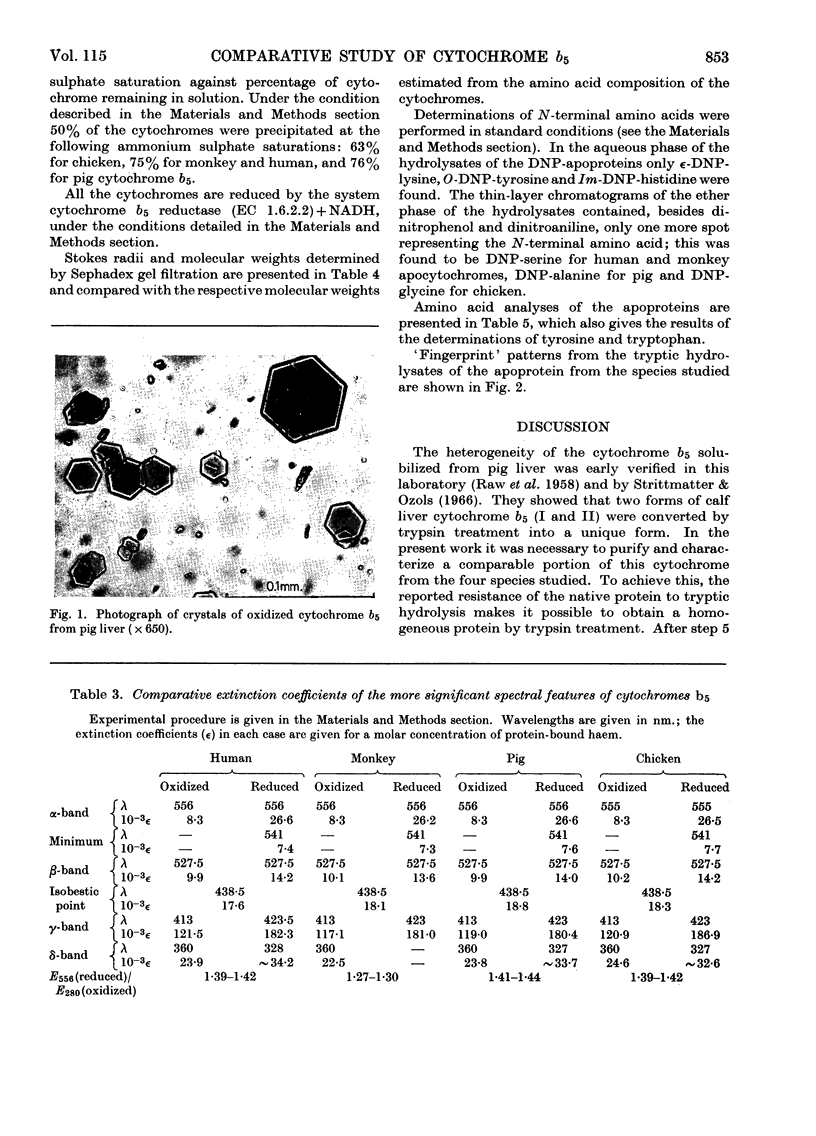
Full text
PDF


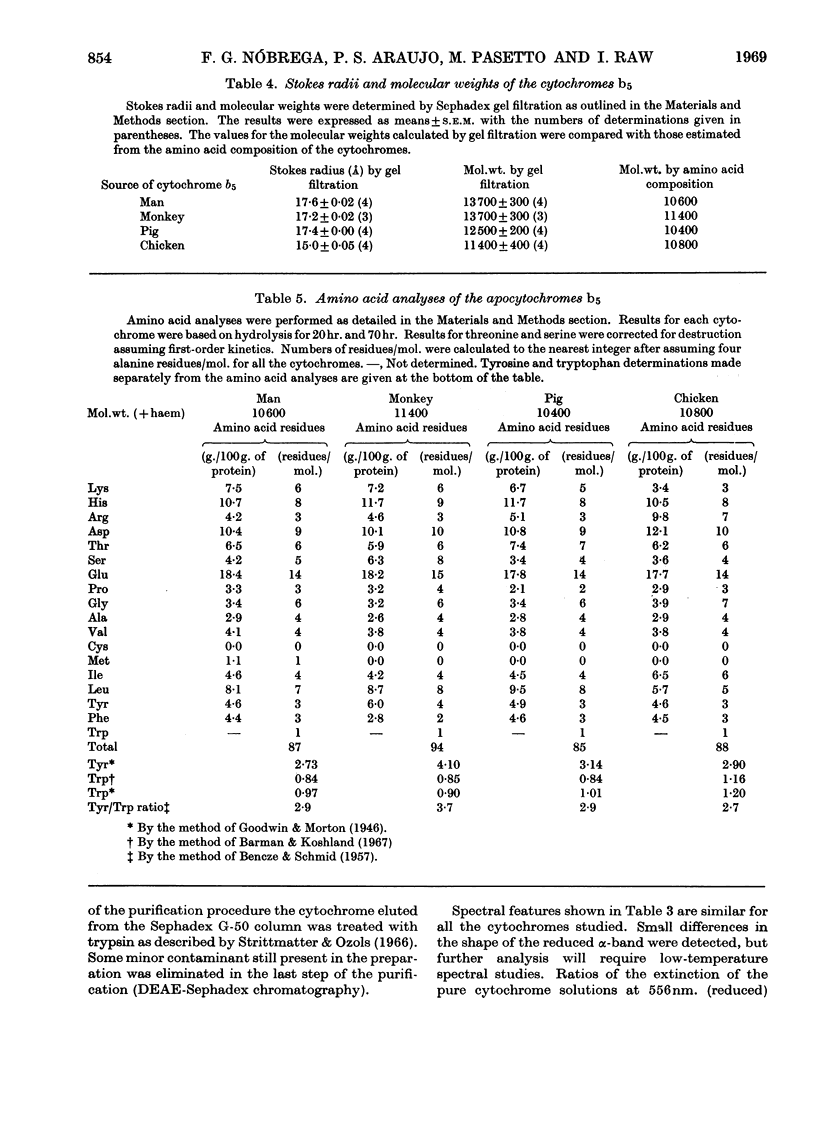

Images in this article
Selected References
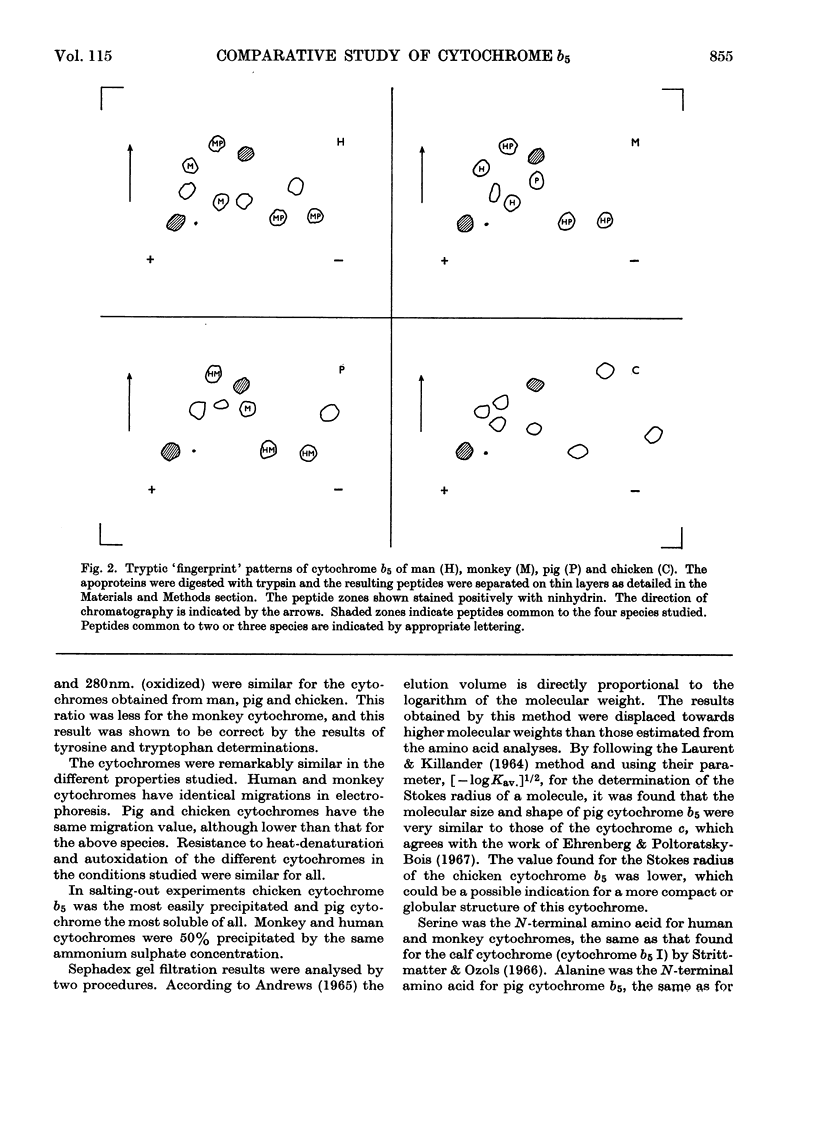
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEBY C. A., MORTON R. K. Lactic dehydrogenase and cytochrome b2 of baker's yeast. Enzymic and chemical properties of the crystalline enzyme. Biochem J. 1959 Nov;73:539–550. doi: 10.1042/bj0730539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman T. E., Koshland D. E., Jr A colorimetric procedure for the quantitative determination of tryptophan residues in proteins. J Biol Chem. 1967 Dec 25;242(23):5771–5776. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- GARFINKEL D. Isolation and properties of cytochrome b5 from pig liver. Arch Biochem Biophys. 1957 Sep;71(1):111–120. doi: 10.1016/0003-9861(57)90012-7. [DOI] [PubMed] [Google Scholar]
- GARFINKEL D. Preparation and properties of a microsomal DPNH-cytochrome c reductase. Arch Biochem Biophys. 1957 Sep;71(1):100–110. doi: 10.1016/0003-9861(57)90011-5. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAHLER H. R., RAW I., MOLINARI R., DO AMARAL D. F. Studies of electron transport enzymes. II. Isolation and some properties of a cytochrome-specific reduced diphosphopyridine nucleotide dehydrogenase from pig liver. J Biol Chem. 1958 Jul;233(1):230–239. [PubMed] [Google Scholar]
- Ozols J., Strittmatter P. The amino acid sequence of cytochrome b-5. J Biol Chem. 1968 Jun 25;243(12):3376–3381. [PubMed] [Google Scholar]
- Ozols J., Strittmatter P. The amino acid sequence of the truptic peptides from cytochrome b5. J Biol Chem. 1968 Jun 25;243(12):3367–3375. [PubMed] [Google Scholar]
- Ozols J., Strittmatter P. The homology between cytochrome B-5, hemoglobin, and myoglobin. Proc Natl Acad Sci U S A. 1967 Jul;58(1):264–267. doi: 10.1073/pnas.58.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLTORATSKY-BOIS R., CHAIX P. NOUVEAU PROC'ED'E DE PURIFICATION DU CYTOCHROME B-5 DE LA CELLULE H'EPATIQUE. Bull Soc Chim Biol (Paris) 1964;46:867–880. [PubMed] [Google Scholar]
- RAW I., MAHLER H. R. Studies of electron transport enzymes. III. Cytochrome b5 of pig liver mitochondria. J Biol Chem. 1959 Jul;234(7):1867–1873. [PubMed] [Google Scholar]
- RAW I., MOLINARI R., DO AMARAL D. F., MAHLER H. R. Studies of electron transport enzymes. I. The purification of cytochrome 556 from pig liver. J Biol Chem. 1958 Jul;233(1):225–229. [PubMed] [Google Scholar]
- STRITTMATTER P., VELICK S. F. The isolation and properties of microsomal cytochrome. J Biol Chem. 1956 Jul;221(1):253–264. [PubMed] [Google Scholar]
- Sargent J. R., Vadlamudi B. P. Characterization and biosynthesis of cytochrome b(5) in rat liver microsomes. Biochem J. 1968 May;107(6):839–849. doi: 10.1042/bj1070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter P., Ozols J. The restricted tryptic cleavage of cytochrome b5. J Biol Chem. 1966 Oct 25;241(20):4787–4792. [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Tsugita A., Kobayashi M., Kajihara T., Hagihara B. Primary structure of rabbit liver cytochrome b5. J Biochem. 1968 Nov;64(5):727–730. doi: 10.1093/oxfordjournals.jbchem.a128954. [DOI] [PubMed] [Google Scholar]
- VESSELINOVITCH S. D. A simple method for making starch-gel electrophoretic strips transparent. Nature. 1958 Sep 6;182(4636):665–665. doi: 10.1038/182665a0. [DOI] [PubMed] [Google Scholar]



