ABSTRACT
Background
Sarcopenia and frailty are often overlooked in assessing kidney transplant (KT) candidates with chronic kidney disease (CKD), potentially leading to poor post-transplant outcomes. This study aimed to identify metabolites associated with frailty and sarcopenia in KT candidates from the FRAILMar study.
Methods
Between June 2016 and June 2020, we evaluated frailty and sarcopenia in 173 KT candidates using the Physical Frailty Phenotype and EGWSOP-2 criteria, respectively. Seventy-five metabolic markers from targeted pathways, previously linked to CKD, sarcopenia or frailty, were measured in serum samples. These markers were analyzed using adjusted and weighted generalized linear models. Metabolomic data were integrated with multi-modal data, such as comorbidities, using a factor-based integration algorithm to identify metabolic phenotypes.
Results
Increased metabolites related to energy metabolism and essential amino acids were associated with frailty, mainly Krebs cycle intermediates. Sarcopenic KT candidates showed lower levels of aromatic amino acids, and lower protein/muscle metabolism, energy metabolism and neurotransmission compared with non-sarcopenic patients. Unsupervised multi-modal integration revealed a high-risk metabolic phenotype characterized by the presence of sarcopenia, diabetes mellitus and low body mass index, with alterations in branched-chain amino acids and high activity of lactate dehydrogenase enzyme.
Conclusions
Frailty and sarcopenia are common among KT candidates, and their metabolic status reveals notable disruptions in energy and amino acid metabolism. These findings highlight the value of a detailed metabolic assessment to more accurately evaluate patient health status prior to transplantation.
Keywords: chronic kidney disease, frailty, metabolomics, sarcopenia, transplant
KEY LEARNING POINTS.
What was known:
Sarcopenia and frailty may be comorbidities of chronic kidney disease that are overlooked in kidney transplant (KT) candidates.
This study adds:
We presented a metabolic fingerprint of sarcopenia and frailty in KT candidates.
We highlighted a high-risk metabolic phenotype in which surgery may be discouraged.
Potential impact:
Patient's metabolic status should be considered when planning surgery in order to enhance kidney transplant success rates.
INTRODUCTION
Kidney transplantation (KT) is the preferred treatment for advanced chronic kidney disease (CKD), particularly in elderly patients. In Spain, 60% of new dialysis patients are over 65 years old, and 20% of 2022 transplant recipients were aged over 70 years [1]. However, older adults are often underrepresented on waiting lists, with only 5% of those aged over 75 years listed for transplantation [2]. This is due to significant morbidity, which frequently coexists with frailty, malnutrition and dependence, which can reduce the value of KT in terms of patient survival and quality of life [3]. While the KT candidate pool is aging, it is crucial to determine whether medical stressors such as transplantation are more likely to negatively affect individuals. Frailty and sarcopenia are critical geriatric syndromes frequently overlooked in KT candidate evaluations. They have overlapping causes and consequences with major impact in this specific clinical setting.
Frailty is characterized by reduced physical functioning and increased vulnerability to adverse health outcomes [4], affecting approximately 20% of KT candidates and linked to poor outcomes during and after transplantation [5]. Despite its clinical relevance, frailty assessment is not routinely performed, and assessment tools vary widely [6]. Sarcopenia, a disorder marked by accelerated muscle loss and function decline [7], affects around 26% of KT recipients [8], and is associated with adverse outcomes in CKD [9], although its impact on KT is not yet fully understood.
CKD's metabolic complications contribute to the development of sarcopenia and frailty [6]. While frailty is theoretically well-characterized, its clinical application remains controversial due to phenotypic heterogeneity and fluctuating severity [10, 11]. Sarcopenia, often influenced by age, genetics and lifestyle, is exacerbated in CKD by factors such as malnutrition, low physical activity and chronic inflammation, especially in older dialysis patients [12]. Assessing these comorbidities in KT candidates could deepen our understanding of their underlying physiological profiles. Metabolic markers reflecting disruptions in amino acid and energy pathways may provide insight into disease severity, enabling more precise interventions [13]. As metabolic signatures gain use as indicators of systemic dysfunction, they offer potential for improved patient management in CKD and KT settings [14]. Metabolic signatures are being used in hospitals and clinical environments to diagnose, select treatments, predict outcomes and aid decision-making [15]. This study aimed to identify metabolomic profiles specifically associated with frailty and sarcopenia in KT candidates, guiding patient-specific interventions for enhanced outcomes.
MATERIALS AND METHODS
Study design and subjects
The FRAILMar study is a prospective cohort study carried out at Hospital del Mar, Barcelona, Spain (NCT04811417). Between June 2016 and June 2020, 455 advanced CKD KT candidates were evaluated for frailty and sarcopenia. Detailed information about the study protocol and the definition of frailty and sarcopenia can be found in the Supplementary Material and Methods.
We undertook a cross-sectional study of the FRAILMar study and chose to assess all available plasma samples. We were able to collect and store samples in 173 patients out of 455 who were evaluated for frailty before transplantation. The Institutional Review Board of Hospital del Mar approved the study, and all enrolled participants provided written informed consent. The study followed the principles of the Declaration of Helsinki, only relying on the official center database.
Frailty assessment
Frailty was assessed according to the Fried scale [16] during KT waiting list evaluation. This scale includes five components: shrinking (unintentional weight loss of ≥4.5 kg), weakness [grip strength below sex- and body mass index (BMI)-specific cut-offs], exhaustion (self-reported), low activity (kilocalories below cut-off) and slowed walking speed (time to walk 4.5 m above cut-off). Each component scores 0 or 1. Robust patients score 0, pre-frail score 1–2 and frail score ≥3.
Sarcopenia assessment
Sarcopenia was diagnosed based on the European Working Group on Sarcopenia in Older People (EWGSOP2) definition, which requires reduced muscle mass and strength [7]. Muscle mass was measured using bioimpedance spectroscopy, with values <80% of reference data considered reduced. Muscle strength was assessed using a handgrip dynamometer (JAMAR, UK), recording the highest value of three attempts (variability <10%). Handgrip strength <27 kg for men and <16 kg for women indicates reduced strength. Confirmed sarcopenia requires both reduced muscle mass and strength.
Other assessments
Additional assessments included self-reported adherence to pharmacological treatment, measured by the four-item Morisky–Green–Levine Medication Adherence Scale [17]. We evaluated instrumental activities of daily living using the Lawton–Brody scale, considering scores <8 in women and <5 in men as indicative of disability [18]. The risk of malnutrition was assessed with the Simplified Nutritional Appetite Questionnaire (SNAQ), where scores of 14 or below indicated potential malnutrition risk [19].
Sample collection and metabolomics analysis
Serum samples from KT candidates were collected at the time of the waiting list evaluation, and frozen at –80°C until analysis. Metabolic pathways were selected based on previously described metabolic pathways altered in CKD, frailty or sarcopenia. Thus, pathways related to amino acid metabolism, inflammation, lipid disposition and energetic production were selected and assessed, using previously reported methods (Supplementary Material and Methods). A panel of 75 targeted biomarkers (Supplementary data, Table S1) was measured by liquid chromatography coupled to tandem mass spectrometry, consisting of an Acquity UPLC instrument (Waters Associates) coupled to a triple quadrupole (TQS Micro, Waters) mass spectrometer. Targeted analytes were determined by selected reaction monitoring. MassLynx software V4.1 (Waters Associates) was used for peak integration and data management.
Statistical analysis
Extended statistical analyses can be found in the Supplementary Material and Methods Baseline assessments included demographics and clinical data, expressed through various statistical methods. Comparisons between groups were made using appropriate statistical tests based on variable types.
The Shapiro–Wilk test was used to assess normality. The metabolomics dataset was log-transformed before modeling. Markers were tested utilizing generalized linear models (GLMs). Potential heterogeneity and imbalance in the population were controlled utilizing a weighted modality of GLMs. For frailty, the Gaussian family was employed during regression, utilizing the frailty score (0 to 5) as an ordinal response. The binomial (logistic) family for dichotomic response was set to assess the presence (yes/no) of sarcopenia. We calculated odd ratios (ORs) from logistic models. Population characteristics were controlled by adjusting for age, sex, BMI, diabetes mellitus and family/social support. The modality of renal replacement therapy (RRT) was specially considered and adjusted during modeling. The Benjamini–Hochberg procedure was applied to control the false-discovery rate (FDR).
Additionally, a multi-omics factor analysis (MOFA+) was performed to extract inherent factors capturing various sources of variability [20]. This dimensionality reduction technique integrated metabolomics and comorbidity data, allowing the correlation of factors with phenotypes at risk of sarcopenia and frailty. Metabolites with loading weights above |0.75| were considered noteworthy. Spearman correlation analyses were conducted to explore relationships between obtained factors and metabolic markers. The comprehensive approach aimed to understand the complex interplay of factors contributing to sarcopenia and frailty in CKD and KT candidates.
RESULTS
Description of the cohort
One hundred and seventy-three KT candidates (72.3% male, mean age 60.7 ± 13.1 years) were evaluated. Among them, 106 patients were on hemodialysis, 42 on peritoneal dialysis and 25 were preemptive candidates. Baseline characteristics are presented in Table 1. Tables 2 and 3 display sociodemographic and clinical features of the cohort according to frailty and sarcopenia status.
Table 1:
Baseline characteristics of KT candidates.
| N = 173 | |
|---|---|
| Sociodemographics | |
| Age (years), mean ± SD | 60.7 ± 13.1 |
| Sex, female, n (%) | 48 (27.7) |
| Caucasian, n (%) | 165 (95.4) |
| Education, no/primary, n (%) | 116 (67.1) |
| Deficient family support, n (%) | 23 (13.3) |
| Socioeconomic status (non-regular incomes), n (%) | 14 (8.1) |
| Grip strength (kg), mean ± SD | 27.99 ± 9.14 |
| Lean body mass, mean ± SD | 14.59 ± 3.97 |
| Fat body mass, mean ± SD | 13.40 ± 7.83 |
| Albumin (g/dL), mean ± SD | 4.24 ± 0.44 |
| Comorbidities | |
| Hypertension, n (%) | 167 (96.5) |
| Diabetes mellitus, n (%) | 66 (38.2) |
| Heart failure, n (%) | 8 (4.6) |
| Ischemic coronary disease, n (%) | 24 (13.9) |
| Peripheral vasculopathy, n (%) | 13 (7.5) |
| Cerebral vasculopathy, n (%) | 11 (6.4) |
| Chronic obstructive pulmonary disease, n (%) | 13 (7.5) |
| RRT modality, n (%) | |
| Hemodialysis | 106 (61.3) |
| Peritoneal dialysis | 42 (24.3) |
| Preemptive candidate | 25 (14.5) |
| Sarcopenia assessment | |
| Sarcopenia according to EWGSOP2 criteria, n (%) | 47 (27.2) |
| Frailty | |
| Frailty prevalence according to Fried scale, n (%) | |
| 0 | 58 (33.5) |
| 1 | 75 (43.4) |
| 2 | 25 (14.5) |
| 3 | 14 (8.1) |
| 4 | 1 (0.6) |
| 5 | 0 |
| Questionnaires | |
| SNAQ >14, n (%) | 114 (65) |
| Lawton–Brody index, median (IQR) | 7 (2) |
| Morisky–Green test, n positive (%) | 139 (80) |
SD, standard deviation; IQR, interquartile range.
Table 2:
Baseline characteristics of KT candidates according to frailty status.
| Fried ≥1, n = 115 | Fried = 0, n = 58 | P-value | |
|---|---|---|---|
| Sociodemographics | |||
| Age (years), mean ± SD | 61.0 ± 14.1 | 60 ± 11.2 | .647 |
| Sex, female, n (%) | 37 (77) | 11 (23) | .047 |
| Caucasian, n (%) | 107 (93) | 58 (100) | .121 |
| Education, no/primary, n (%) | 85 (73.9) | 31 (53.4) | .063 |
| Deficient family support, n (%) | 20 (17.4) | 3 (5.2) | .032 |
| Socioeconomic status, non-regular incomes, n (%) | 9 (7.9) | 5 (8.6) | .541 |
| Albumin (g/dL), mean ± SD | 4.24 ± 0.46 | 4.22 ± 0.41 | .795 |
| Comorbidities | |||
| Hypertension, n (%) | 112 (97.4) | 55 (96.5) | .537 |
| Diabetes mellitus, n (%) | 42 (36.5) | 24 (42.1) | .293 |
| Heart failure, n (%) | 6 (5.2) | 2 (3.4) | .461 |
| Ischemic coronary disease, n (%) | 15 (13) | 9 (15.5) | .410 |
| Peripheral vasculopathy, n (%) | 10 (8.7) | 3 (5.2) | .309 |
| Cerebral vasculopathy, n (%) | 5 (4.3) | 6 (10.3) | .118 |
| COPD, n (%) | 10 (8.7) | 3 (5.2) | .309 |
| Hemodialysis as RRT modality, n (%) | 77 (67) | 29 (50) | .023 |
| Sarcopenia assessment | |||
| Sarcopenia according to EWGSOP2 criteriaa, n (%) | 47 (40.9) | 0 | <.001 |
aEleven out of 15 frail patients were sarcopenic, and 36 out of 100 pre-frail patients.
COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Table 3:
Baseline characteristics of KT candidates according to sarcopenia status.
| Sarcopenia, n = 47 | No sarcopenia, n = 126 | P-value | |
|---|---|---|---|
| Sociodemographics | |||
| Age (years, mean ± SD) | 65.8 ± 11.3 | 58.8 ± 13.3 | .001 |
| Sex, female, n (%) | 20 (42.6) | 28 (22.2) | .012 |
| Caucasian, n (%) | 44 (93.6) | 121 (96) | .309 |
| Education, no/primary, n (%) | 38 (80.5) | 78 (61.9) | .090 |
| Deficient family support, n (%) | 12 (25.5) | 11 (8.7) | .010 |
| Socioeconomic status, non-regular incomes, n (%) | 4 (8.7) | 10 (7.9) | .872 |
| Albumin (g/dL), mean ± SD | 4.17 ± 0.56 | 4.27 ± 0.39 | .277 |
| Comorbidities | |||
| Hypertension, n (%) | 46 (97.9) | 121 (96.8) | .709 |
| Diabetes mellitus, n (%) | 23 (48.9) | 43 (34.4) | .081 |
| Heart Failure, n (%) | 2 (4.3) | 6 (4.8) | .888 |
| Ischemic coronary disease, n (%) | 6 (12.8) | 18 (14.3) | .797 |
| Peripheral vasculopathy, n (%) | 6 (12.8) | 7 (5.6) | .118 |
| Cerebral vasculopathy, n (%) | 3 (6.4) | 8 (6.3) | .994 |
| COPD, n (%) | 4 (8.5) | 9 (7.1) | .761 |
| Hemodialysis as RRT modality, n (%) | 33 (70.2) | 73 (57.9) | .140 |
| Frailty assessment | |||
| Fried scale = 1 or 2 criteria, n (%) | 36 (76.6) | 64 (50.8) | .003 |
| Fried scale ≥3 criteria; n (%) | 11 (23.4%) | 4 (3.2%) | <.001 |
COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Metabolic features associated with frailty and sarcopenia
Our results showed that 10 metabolites were associated with frailty with a nominal P-value <.05, as shown in Table 4. However, only malate, lactate, succinate and methionine remained significantly associated with frailty after FDR correction. Slopes of the models indicated that the concentration of these metabolites increased with the frailty score. Twenty metabolites were associated with sarcopenia with a nominal P-value <.05 and are presented in Table 5. Odds ratios and the 95% confidence interval of each metabolic feature are illustrated in Fig. 1. After the application of the FDR correction, six molecules remained significantly associated with sarcopenic KT candidates. Relative to non-sarcopenic patients, a lower concentration of metabolites related to protein/muscle metabolism (i.e. phenylalanine, tyrosine and creatinine), energy metabolism (i.e. carnitine) and neurotransmitter metabolism (i.e. serotonin and tryptophan) were found in sarcopenic KT candidates.
Table 4:
Metabolic associations (ng/mL) with frailty score in KT candidates.
| Metabolite | Slopea (95% CI) | P-valuea | FDR |
|---|---|---|---|
| Lactate | 0.42 (0.19–0.65) | <.001 | 0.017 |
| Malate | 0.44 (0.20–0.68) | <.001 | 0.017 |
| Methionine | 0.72 (0.30–1.15) | .001 | 0.026 |
| Succinate | 0.61 (0.24–0.98) | .002 | 0.028 |
| Creatinine | –0.52 (–0.89 to –0.14) | .008 | 0.116 |
| Malate/Fumarate | 0.58 (1.14–1.03) | .011 | 0.140 |
| Citrate/Malate | –0.29 (–0.51 to –0.06) | .015 | 0.158 |
| Fumarate | 0.29 (0.03–0.55) | .028 | 0.266 |
| Acetoacetate | –0.14 (–0.26 to –0.01) | .032 | 0.268 |
| Glutamate/Glutamine | 0.23 (0.01–0.45) | .045 | 0.335 |
Slope and P-values from weighted generalized linear models using the frailty score (numeric, 0–5) as response.
Models were adjusted for by age, sex, BMI, diabetes mellitus, type of renal replacement therapy and family/social support.
CI, confidence interval; FDR, false-discovery-rate corrected P-values; SD, standard deviation.
Table 5:
Metabolic associations (ng/mL) with sarcopenia in KT candidates.
| Metabolite | Controls (mean ± SD) | Sarcopenia (mean ± SD) | OR (95% CI)a | P-valuea | FDR |
|---|---|---|---|---|---|
| Phenylalanine | 20 288.2 ± 6286.8 | 17 859.6 ± 4874.3 | 0.19 (0.06–0.51) | .001 | 0.044 |
| Creatinine | 45 327.3 ± 18096.1 | 36 146.5 ± 11637.2 | 0.25 (0.1–0.58) | .002 | 0.044 |
| Serotonin | 53.28 ± 36.32 | 42.06 ± 36.63 | 0.59 (0.42–0.82) | .002 | 0.044 |
| Tyrosine | 8856.4 ± 3265.8 | 8480.5 ± 3068.4 | 0.26 (0.1–0.62) | .003 | 0.044 |
| Carnitine | 4780.5 ± 4151.8 | 3990.7 ± 5382.7 | 0.43 (0.24–0.75) | .003 | 0.044 |
| Tryptophan | 5489.9 ± 1505.1 | 5040.2 ± 1461.4 | 0.21 (0.07–0.59) | .004 | 0.044 |
| Hippuric acid | 24.16 ± 24.05 | 42.05 ± 42.10 | 1.57 (1.15–2.17) | .005 | 0.057 |
| MAO | 0.325 ± 0.607 | 0.447 ± 0.558 | 1.48 (1.13–1.98) | .006 | 0.057 |
| BCAA/AAA | 0.985 ± 0.186 | 1.02 ± 0.22 | 7.62 (1.64–37.71) | .011 | 0.089 |
| 20a-DHF | 8.451 ± 5.652 | 7.884 ± 3.93 | 0.54 (0.32–0.86) | .013 | 0.097 |
| Cortisol | 185.48 ± 83.83 | 183.3 ± 73.51 | 0.46 (0.24–0.84) | .014 | 0.098 |
| 5a-THF | 59.78 ± 39.87 | 46 ± 26.91 | 0.56 (0.33–0.93) | .026 | 0.148 |
| Pyruvate | 34.55 ± 55.84 | 18.66 ± 12.86 | 0.64 (0.41–0.95) | .031 | 0.148 |
| 3OH-Kynurenine/kynurenine | 0.029 ± 0.013 | 0.032 ± 0.012 | 2.01 (1.09–3.91) | .031 | 0.148 |
| Leucine | 13 143.6 ± 3646.5 | 12 318.8 ± 3760.9 | 0.31 (0.1–0.89) | .031 | 0.148 |
| Kynurenine | 642.22 ± 229.06 | 613.25 ± 189.8 | 0.36 (0.14–0.9) | .032 | 0.148 |
| Acetylcarnitine | 1238.2 ± 1104.1 | 984.6 ± 1214.1 | 0.57 (0.34–0.96) | .034 | 0.148 |
| MA/FA | 12.35 ± 4.241 | 10.884 ± 3.345 | 0.41 (0.17–0.93) | .036 | 0.149 |
| LDH | 3.975 ± 3.636 | 4.66 ± 4.928 | 1.56 (1.03–2.43) | .042 | 0.164 |
| Glutamate | 36.16 ± 21.49 | 28.95 ± 13.35 | 0.56 (0.31–0.99) | .047 | 0.177 |
ORs and P-values from weighted logistic models with binomial distribution utilizing presence of sarcopenia (binary, yes/no) as response.
Models were adjusted for by age, sex, BMI, diabetes mellitus, type of RRT and family/social support.
CI, confidence interval; FDR, false-discovery-rate corrected P-values; SD, standard deviation.
Figure 1:
Association Polar plot illustrating odd ratios (ORs) and 95% confidence intervals from weighted generalized linear models with binomial distribution, adjusted for described covariates (see Statistical analysis section), for the association of risk biomarkers of sarcopenia. The left legend displays the OR values at the corresponding inner circle. Dotted circle indicates an OR of 1. Colored HR boxes indicate nominal P-value <.05. 11HSD, 11β-hydroxysteroid dehydrogenase; 20a-DHE, 20α-dihydrocortisone; 20a-DHF, 20α-dihydrocortisol; 20b-DHE, 20β-dihydrocortisone; 20b-DHF, 20β-dihydrocortisol; 3OHkyn, 3-hydroxykynurenine; 5a-THF, 5α-tetrahydrocortisol; 5b-THF, 5β-tetrahydrocortisol; 5HT, serotonin; 6OH-F, 6β-hydroxycortisol; aKG, α-ketoglutarate; CA, citric acid/citrate; FA, fumaric acid/fumarate; Gln, glutamine; Glu, glutamate; IDO, indoleamine 2,3-dioxygenase (kynurenine/tryptophan ratio); Kyn, kynurenine; LDH, lactate dehydrogenase (lactate/pyruvate ratio); MA, malic acid/malate; MAO, monoamine oxidase (5-hydroxy indoleacetic acid/serotonin ratio); SA, succinic acid/succinate; Trp, tryptophan; OH, hydroxy.
Unsupervised multi-modal integration revealed underlying metabolic phenotypes
This study employed unsupervised multi-modal integration to cluster metabolomics, sarcopenia, frailty, RRT, BMI, sex, age, diabetes mellitus and family support data into eight distinct factors, shown in Supplementary data, Fig. S1 The strongest association was observed between factor 2 and RRT (P < .001), followed by factor 3, correlating with BMI (P < .001), diabetes mellitus (P = .005) and sarcopenia (P = .020). Factor 1 exhibited a correlation with frailty (P = .035), while factor 8 correlated with BMI, and factor 5 with sex and RRT. Factor 5 metabolites revealed the metabolic status of KT candidates based on RRT and sex. Factors 6 and 8 showed no association with the studied covariates, and their etiology remains unknown.
Further exploration focused on factor 1 due to its moderate correlation with frailty, and factor 3, representing a metabolic phenotype related to comorbidities (sarcopenia, diabetes mellitus and low BMI) in KT candidates. Metabolites’ scaled loading-weights from factor 1 and unadjusted Spearman correlation coefficients between metabolites and frailty are presented in Supplementary data, Fig. S2 Notably, malate and lactate, associated with frailty, were positively linked to factor 1, while the citrate/malate ratio exhibited an inverse association.
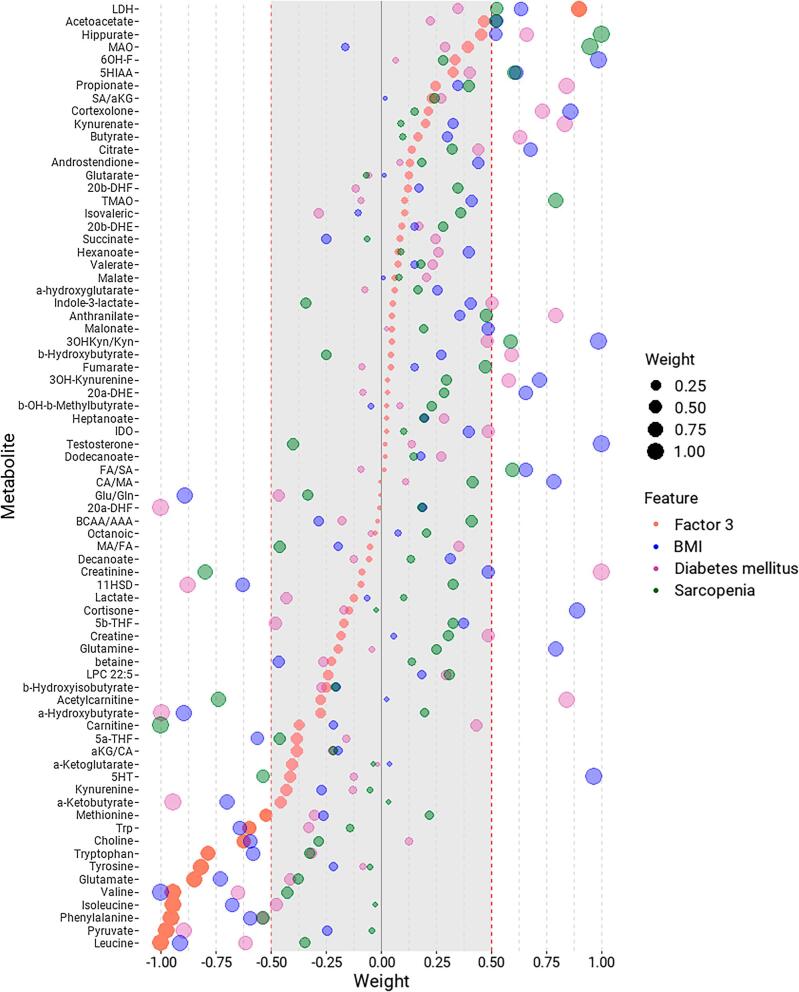
In correlation analyses, the enzyme monoamine oxidase (MAO) and carnitine displayed a strong association with frailty but not with factor 1. Similarly, the metabolites associated with sarcopenia, diabetes mellitus and low BMI are presented in Fig. 2. In factor 3, branched-chain amino acids (BCAAs; leucine, isoleucine, valine), aromatic amino acids (AAAs; phenylalanine, tryptophan, tyrosine), glutamate and pyruvate had the lowest loading weights. Patients with sarcopenia, diabetes mellitus and low BMI exhibited low concentrations of these metabolites. Lactate dehydrogenase (LDH) increased with factor 3 and positively correlated with low BMI and sarcopenia, suggesting its relevance in this phenotype.
Figure 2:
Metabolites’ loading weights from factor 3 and scaled correlation factors of metabolic markers with BMI, diabetes mellitus and sarcopenia. Metabolites are sorted by their loading-weights in factor 3, shown in orange. Loading weights above 0.75 denoted positive correlations with diabetes mellitus, sarcopenia and low BMI, while loading weights below –0.75 implied inverse correlations with such features. Spearman correlation factors from correlations between metabolites and diabetes mellitus (pink) and sarcopenia (green) were scaled between –1 and 1. For consistency, Spearman correlation factors from correlations between metabolites and BMI were multiplied by –1, meaning positive associations with low BMI (blue).
DISCUSSION
Our results suggest that there are a few metabolites related to energy metabolism and essential amino acids with the presence of frailty in KT candidates. Lactate is produced during anaerobic energy production, while succinate and malate are part of the aerobic Krebs cycle. Disruptions in aerobic metabolism, such as mitochondrial dysfunction, can lead to lactate accumulation [21]. The increase of malate, lactate, pyruvate and glutamate, and the decrease of the ratio citrate/malate, shown in factor 1, support these alterations in energy metabolism. The kidneys, being highly metabolic organs with abundant mitochondria, require significant ATP [22]. Disruption of mitochondrial homeostasis during acute kidney injury leads to tubular injury and persistent renal dysfunction [23]. In CKD, comorbidities such as diabetes mellitus disrupt the supply of essential metabolic substrates, including oxygen and macronutrients, affecting ATP production and altering metabolic fuel sources [24]. An accumulation of lactic acid may indicate imbalances in energy production and utilization, which can impact cellular health [21] and lead to potential disturbances in cellular metabolism in frail KT candidates.
While limited research exists on the metabolomics of frail and sarcopenic CKD patients, several studies have identified distinct metabolite biomarkers associated with CKD progression and related complications. For instance, Rhee et al. found that uric acid and glucuronate levels were elevated in patients with rapidly declining estimated glomerular filtration rate, while amino acids like threonine, methionine phenylalanine and arginine were decreased, indicating metabolic dysregulation [25]. Kimura et al. highlighted a composite predictive value for CKD progression using metabolites such as trimethylamine N-oxide (TMAO) and gluconate in patients not on dialysis [26]. Additionally, Lee et al. reported increased TMAO and creatinine levels, coupled with decreased essential amino acids in pre-dialysis CKD patients compared with healthy controls [27]. The presence of diabetes more strongly affected the metabolic signature during early-stage CKD.
Regarding frailty, recent studies emphasize the potential of metabolomics in revealing the metabolic changes underlying frailty [28]. The interaction among aging, frailty and metabolism is actively researched, shaping the health trajectory of older adults. The FRAILOMIC initiative found a cross-sectional association between plasma 3-methylhistidine concentrations and frailty in adults aged 65 years and older [29]. In the European METABOFRAIL study, in addition to replicating higher circulating 3-methylhistidine, amino acid–related metabolites such as alanine, arginine, glutamic acid, sarcosine, tryptophan and ethanolamine were found to be higher in 66 older diabetic adults who were either frail/pre-frail compared with 30 robust non-diabetic controls [30]. Meng and coworkers described lower tryptophan and higher glycine levels in frail older men, but tryptophan was also associated with overlapping frailty and sarcopenia in this population [31]. In the BIOSPHERE study, Calvani and colleagues described higher levels of asparagine, aspartate, citrulline, ethanolamine, glutamate, sarcosine and taurine in older adults with both physical frailty and sarcopenia [32]. In another study assessing biomarkers for biological age with mention to frailty risk, the authors associated a set of 14 biomarkers, composed of three lipoproteins, the ratio of polyunsaturated fatty acids to total fatty acids, glucose, lactate, histidine, phenylalanine, acetoacetate, albumin, the three BCAAs and glycoprotein acetyls, with frailty [33]. In the present study, none of these compounds were dysregulated in frail KT candidates.
Our results are partially in line with a study on the frailty in Chinese older adults, in which the Krebs cycle-related metabolites such as malate, fumarate and cis-aconitate were described as potential biomarkers for early diagnosis of frailty in non-CKD patients [34]. The scarce evidence may be due to the complex, progressive and multifactorial pathophysiology and etiopathogenesis of frailty occurring with different comorbidities [35].
On the contrary, there is increasing evidence that changes in metabolism may play a key role in the development and progression of sarcopenia [14]. While the clinical presentation of frailty and sarcopenia are similar in that both conditions involve a reduction in muscle mass and strength/function as individuals age [36], there seems to be a difference in the metabolic process. Our results suggest changes in creatinine, carnitine, AAA metabolism and energetic metabolism.
While plasma creatinine was statistically significant, its levels in dialysis patients can be strongly influenced by the timing of sample collection, potentially introducing bias due to fluctuations tied to dialysis schedules. Nonetheless, lower creatinine levels have been previously linked to muscle atrophy or reduced muscle mass, which are core characteristics of sarcopenia [37]. Low carnitine levels can impair energy production in muscle cells, contributing to muscle weakness, and this was proposed as a potential biomarker of sarcopenia in a cohort of 114 gastrointestinal cancer patients [38]. In another study, patients with heart failure and serum carnitine <36 μmol/L showed more muscle weakness and a shorter 6-min walk distance [39]. In sarcopenic KT candidates, altered metabolism of AAAs like phenylalanine, tyrosine and tryptophan indicates dietary deficiencies or impaired metabolism, contributing to muscle loss. Reduced tryptophan, crucial for serotonin synthesis, combined with higher MAO levels, may contribute to lower serotonin. This can lead to mood, appetite and well-being issues, worsening sarcopenia symptoms. However, it is important to note that our study did not collect data on patients’ medications or psychiatric comorbidities, both of which could impact plasma serotonin levels. Therefore, findings should be interpreted with caution. Low levels of serum tryptophan were also associated with skeletal muscle atrophy in patients with B-cell lymphoma [40]. Sol et al., recently outlined dysregulations in compounds related to lipid and AAA metabolisms as the main metabolic pathways affected by age, with a relevant influence of sex [41]. Several gut-derived AAA catabolites were found in the authors’ study, suggesting that changes in the microbiota during chronological aging might contribute to age-related disorders [42]. In this sense, lower tryptophan levels and a higher 3-hydroxykynurenine/kynurenine ratio indicate a shift in tryptophan metabolism towards kynurenine pathway. This shift is associated with chronic immune activation and dysregulation [43] and may include increased inflammation, oxidative stress and T-cell dysfunction, which can compromise immune homeostasis. In our study, factor 3 characterized sarcopenic KT candidates with diabetes mellitus but low BMI. This phenotype presented an inverse association with all BCAAs and AAAs, and a positive association with LDH, an enzyme involved in the interconversion between lactate and pyruvate during anaerobic metabolism. An inverse association of pyruvate supports this imbalance in favor of lactate.
Growing evidence highlights that impaired BCAA catabolism is crucial in insulin resistance among obese and diabetic individuals. However, variations in BCAA metabolism are noted between obese and diabetic patients, as well as KT candidates with sarcopenia, diabetes and low BMI, emphasizing BCAA loss in this context. Le Couteur and colleagues proposed that despite higher blood BCAA levels correlating with elevated glucose, insulin, HOMA-IR and triglycerides, frail individuals exhibited lower BCAA levels inversely linked to mortality and major cardiovascular events [44]. Nakajima et al. proposed leucine and glutamate as biomarkers of sarcopenic risk in Japanese patients with type 2 diabetes mellitus [45], but the authors did not control for false-positive rate and these findings should be interpreted carefully. Conversely, in a nested case–control study, composed of 79 participants with a low muscle quality and 79 matched controls of the Baltimore Longitudinal Study of Aging, the authors showed elevated circulating levels of tryptophan, serotonin and methionine; and BCAAs leucine and isoleucine in the declined muscle group [46]. Although our results for leucine and the ratio BCAA/AAA could support the association between BCAA and sarcopenia, the FDR correction indicated that the associations are not sufficiently noteworthy without the presence of comorbidities captured by factor 3.
This study is limited by its observational nature. The absence of a control group of patients without CKD and the delay in sample collection after dialysis treatment may have reduced the sensitivity of the analyses. Therefore, data distribution, dispersion and the presence of outliers were carefully investigated to minimize potential inconsistencies and uncontrolled variability in this cross-sectional study.
CONCLUSION
Our analysis of serum metabolites in kidney transplant candidates with sarcopenia and frailty reveals significant disruptions in energy and amino acid metabolism. These findings suggest a distinct metabolic profile associated with frailty and sarcopenia in this patient population. Recognizing these metabolic alterations emphasizes the potential value of individualized metabolic assessment to better characterize candidate health status before transplantation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors of this study appreciate the contribution of all the members of the FRAILMar Study Group.
Notes
Group members in supplementary list.
Contributor Information
Francisco Madrid-Gambin, Applied Metabolomics Research Group, Hospital del Mar Research Institute, Barcelona, Spain.
María José Pérez-Sáez, Nephrology Department, Hospital del Mar, Barcelona, Spain; Nephropathies Research Group, Hospital del Mar Research Institute, Barcelona, Spain.
Alex Gómez-Gómez, Applied Metabolomics Research Group, Hospital del Mar Research Institute, Barcelona, Spain.
Noemí Haro, Applied Metabolomics Research Group, Hospital del Mar Research Institute, Barcelona, Spain.
Dolores Redondo-Pachón, Nephrology Department, Hospital del Mar, Barcelona, Spain; Nephropathies Research Group, Hospital del Mar Research Institute, Barcelona, Spain.
Vanessa Dávalos-Yerovi, Physical Medicine and Rehabilitation Department, Parc de Salut Mar (Hospital del Mar-Hospital de l'Esperança), Rehabilitation Research Group, Hospital del Mar Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain.
Ester Marco, Physical Medicine and Rehabilitation Department, Parc de Salut Mar (Hospital del Mar-Hospital de l'Esperança), Rehabilitation Research Group, Hospital del Mar Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain.
Marta Crespo, Nephrology Department, Hospital del Mar, Barcelona, Spain; Nephropathies Research Group, Hospital del Mar Research Institute, Barcelona, Spain.
Oscar J Pozo, Applied Metabolomics Research Group, Hospital del Mar Research Institute, Barcelona, Spain.
Julio Pascual, Nephrology Department, Hospital del Mar, Barcelona, Spain; Nephropathies Research Group, Hospital del Mar Research Institute, Barcelona, Spain; Nephrology Department, Hospital Universitario 12 de Octubre, Madrid, Spain.
for the FRAILMar Study Group:
María José Pérez-Sáez, Betty Chamoun, Dolores Redondo, Francesc Barbosa, Higini Cao, Silvia Collado, Anna Buxeda, Carla Burballa, Marta Crespo, Julio Pascual, Anna Faura, María Vera, Anna Bach, Guillermo Pedreira, Ernestina Junyent, Montserrat Folgueiras, Yolanda Castillo, Aida Martínez, Marisol Fernández, Eva Barbero, Noelia Fernández, Alicia Calvo, Jesús Carazo, Albert Frances, Lluis Cecchini, Ester Marco, Elena Muñoz, Lou Delcros-Forestier, Delky Meza de Valderrama, Andrea Morgado, Xavier Nogués, Leocadio Rodríguez-Mañas, Olga Vázquez, María Dolores Muns, Miguel Gárriz, María Polo Gómez, Sara Hurtado, Maite López, Laura Ribera, Margarita Guino, Ramón Roca, Jordi Calls, Alicia Rovira, Josep Mora, Omar Ibrik, Florentina Liria, Thaïs López, Jaume Almirall, Carmen Moya, Fátima Moreno, Manel Ramírez de Arellano, Sandra Rubio, Ignacio Cidraque, Carlota Pájaro, Núria Garra, Josep Galcerán, Marina Fenollar, Sara Outón, Josep Jara, Rosa García, and Mònica Manresa
FUNDING
FRAILMar project is currently supported by a FIS-FEDER grant PI19/00 037 (ISCIII). F.M.-G. was supported by Grant FJC2018-035791-I funded by MCIN/AEI/10.13039/501100011033.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author, J.P., upon reasonable request.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Organización Nacional de Trasplantes . Actividad de donación y trasplante renal. España 2023;XII:93. http://www.ont.gob.es/infesp/Memorias/Actividad_de_Donación_y_Trasplante_Renal_2019.pdf%0Ahttp://www.ont.es/infesp/Memorias/Actividad de Donación y Trasplante Renal.pdf [Google Scholar]
- 2.Informe estadístic 2020 del Registre de Malalts Renals de Catalunya (RMRC) . Donació i trasplantament.
- 3.McAdams-Demarco MA, King EA, Luo Xet al. Frailty, length of stay, and mortality in kidney transplant recipients. Ann Surg 2017;266:1084–90. 10.1097/SLA.0000000000002025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cesari M, Prince M, Thiyagarajan JAet al. Frailty: an emerging public health priority. J Am Med Dir Assoc 2016;17:188–92. 10.1016/j.jamda.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 5.Harhay MN, Rao MK, Woodside KJet al. An overview of frailty in kidney transplantation: measurement, management and future considerations. Nephrol Dial Transplant 2020;35:1099–112. 10.1093/ndt/gfaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAdams-DeMarco MA, Van Pilsum Rasmussen SE, Chu NMet al. Perceptions and practices regarding frailty in kidney transplantation: results of a national survey. Transplantation 2020;104:349–56. 10.1097/TP.0000000000002779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Bahat G, Bauer Jet al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. [DOI] [PubMed]
- 9.Ortiz A, Sanchez-Niño MD.. Sarcopenia in CKD: a roadmap from basic pathogenetic mechanisms to clinical trials. Clin Kidney J 2019;12:110–2. 10.1093/ckj/sfz001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesari M, Calvani R, Marzetti E.. Frailty in older persons. Clin Geriatr Med 2017;33:293–303. http://www.geriatric.theclinics.com/article/S0749069017300113/fulltext [DOI] [PubMed] [Google Scholar]
- 11.Stolz E, Mayerl H, Freidl W.. Fluctuations in frailty among older adults. Age Ageing 2019;48:547–52. https://academic.oup.com/ageing/article/48/4/547/5480913 [DOI] [PubMed] [Google Scholar]
- 12.Sabatino A, Cuppari L, Stenvinkel Pet al. Sarcopenia in chronic kidney disease: what have we learned so far? J Nephrol 2021;34:1347–72. 10.1007/s40620-020-00840-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menon MC, Murphy B, Heeger PS.. Moving biomarkers toward clinical implementation in kidney transplantation. J Am Soc Nephrol 2017;28:735–47. https://journals.lww.com/jasn/Fulltext/2017/03000/Moving_Biomarkers_toward_Clinical_Implementation.7.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picca A, Coelho-Junior HJ, Cesari Met al. The metabolomics side of frailty: toward personalized medicine for the aged. Exp Gerontol 2019;126:110692. 10.1016/j.exger.2019.110692 [DOI] [PubMed] [Google Scholar]
- 15.Castelli FA, Rosati G, Moguet Cet al. Metabolomics for personalized medicine: the input of analytical chemistry from biomarker discovery to point-of-care tests. Anal Bioanal Chem 2022;414:759–89. https://link.springer.com/article/10.1007/s00216-021-03586-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LP, Tangen CM, Walston Jet al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–57. [DOI] [PubMed] [Google Scholar]
- 17.Morisky DE, Green LW, Levine DM.. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74. https://pubmed.ncbi.nlm.nih.gov/3945130/ [DOI] [PubMed] [Google Scholar]
- 18.Lawton MP, Brody EM.. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 19.Wilson MMG, Thomas DR, Rubenstein LZet al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr 2005;82:1074–81. https://pubmed.ncbi.nlm.nih.gov/16280441/ [DOI] [PubMed] [Google Scholar]
- 20.Argelaguet R, Arnol D, Bredikhin Det al. MOFA+: a statistical framework for comprehensive integration of multi-modal single-cell data. Genome Biol 2020;21:1–17. https://genomebiology.biomedcentral.com/articles/10.1186/s13059-020-02015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Yang Y, Zhang Bet al. Lactate metabolism in human health and disease. Signal Transduct Target Ther 2022;7:1–22. https://www.nature.com/articles/s41392-022-01151-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duann P, Lin PH.. Mitochondria damage and kidney disease. Adv Exp Med Biol 2017;982:529–51. https://link.springer.com/chapter/10.1007/978-3-319-55330-6_27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhargava P, Schnellmann RG.. Mitochondrial energetics in the kidney. Nat Rev Nephrol 2017;13:629–46. https://www.nature.com/articles/nrneph.2017.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forbes JM, Thorburn DR.. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol 2018;14:291–312. https://www.nature.com/articles/nrneph.2018.9 [DOI] [PubMed] [Google Scholar]
- 25.Rhee EP, Clish CB, Wenger Jet al. Metabolomics of CKD progression: a case-control analysis in the chronic renal insufficiency cohort study. Am J Nephrol 2016;43:366. https://pmc.ncbi.nlm.nih.gov/articles/PMC4880483/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T, Yasuda K, Yamamoto Ret al. Identification of biomarkers for development of end-stage kidney disease in chronic kidney disease by metabolomic profiling. Sci Rep 2016;6:26138. https://pubmed.ncbi.nlm.nih.gov/27188985/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Choi JY, Kwon YKet al. Changes in serum metabolites with the stage of chronic kidney disease: comparison of diabetes and non-diabetes. Clin Chim Acta 2016;459:123–31. https://pubmed.ncbi.nlm.nih.gov/27221201/ [DOI] [PubMed] [Google Scholar]
- 28.Kondoh H, Kameda M.. Metabolites in aging and aging-relevant diseases: frailty, sarcopenia and cognitive decline. Geriatr Gerontol Int 2024;4 Suppl 1(Suppl 1):44–8. https://pubmed.ncbi.nlm.nih.gov/37837183/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochlik B, Stuetz W, Pérès Ket al. Associations of plasma 3-methylhistidine with frailty status in French cohorts of the FRAILOMIC Initiative. J Clin Med 2019;8:1010. 10.3390/jcm8071010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calvani R, Rodriguez-Mañas L, Picca Aet al. Identification of a circulating amino acid signature in frail older persons with type 2 diabetes mellitus: results from the Metabofrail study. Nutrients 2020;12:199. https://www.mdpi.com/2072-6643/12/1/199/htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng L, Shi H, Wang DGet al. Specific metabolites involved in antioxidation and mitochondrial function are correlated with frailty in elderly men. Front Med 2022;9:816045. 10.3389/fmed.2022.816045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calvani R, Picca A, Marini Fet al. A distinct pattern of circulating amino acids characterizes older persons with physical frailty and sarcopenia: results from the BIOSPHERE study. Nutrients 2018;10:1691. https://www.mdpi.com/2072-6643/10/11/1691/htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuiper LM, Polinder-Bos HA, Bizzarri Det al. Epigenetic and metabolomic biomarkers for biological age: a comparative analysis of mortality and frailty risk. J Gerontol Ser A 2023;2023:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan Y, Li Y, Liu Pet al. Metabolomics-based frailty biomarkers in older Chinese adults. Front Med 2022;8:830723. 10.3389/fmed.2021.830723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan Y, Ji T, Li Yet al. Omics biomarkers for frailty in older adults. Clin Chim Acta 2020;510:363–72. 10.1016/j.cca.2020.07.057 [DOI] [PubMed] [Google Scholar]
- 36.Cesari M, Landi F, Calvani Ret al. Rationale for a preliminary operational definition 314of physical frailty and sarcopenia in the SPRINTT trial. Aging Clin Exp Res 2017;29:81–8. https://link.springer.com/article/10.1007/s40520-016-0716-1 [DOI] [PubMed] [Google Scholar]
- 37.Moorthi RN, Avin KG.. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens 2017;26:219–28. 10.1097/MNH.0000000000000318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takagi A, Hawke P, Tokuda Set al. Serum carnitine as a biomarker of sarcopenia and nutritional status in preoperative gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle 2022;13:287–95. https://onlinelibrary.wiley.com/doi/full/10.1002/jcsm.12906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinugasa Y, Sota T, Nakamura Ket al. Association of carnitine insufficiency with sarcopenia and dynapenia in patients with heart failure. Geriatr Gerontol Int 2023;23:524–30. https://onlinelibrary.wiley.com/doi/full/10.1111/ggi.14621 [DOI] [PubMed] [Google Scholar]
- 40.Ninomiya S, Nakamura N, Nakamura Het al. Low levels of serum tryptophan underlie skeletal muscle atrophy. Nutrients 2020;12:978. https://www.mdpi.com/2072-6643/12/4/978/htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sol J, Obis È, Mota-Martorell Net al. Plasma acylcarnitines and gut-derived aromatic amino acids as sex-specific hub metabolites of the human aging metabolome. Aging Cell 2023;22:e13821. https://onlinelibrary.wiley.com/doi/full/10.1111/acel.13821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran SMS, Hasan Mohajeri M.. The role of gut bacterial metabolites in brain development, aging and disease. Nutrients 2021;13:1–41. https://pubmed.ncbi.nlm.nih.gov/33669008/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ballesteros J, Rivas D, Duque G.. The role of the kynurenine pathway in the pathophysiology of frailty, sarcopenia, and osteoporosis. Nutrients 2023;15:3132. https://pubmed.ncbi.nlm.nih.gov/37513550/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Couteur DG, Ribeiro R, Senior Aet al. Branched chain amino acids, cardiometabolic risk factors and outcomes in older Men: the Concord Health and Ageing in Men Project. J Gerontol Ser A 2020;75:1805–10. [DOI] [PubMed] [Google Scholar]
- 45.Nakajima H, Okada H, Kobayashi Aet al. Leucine and glutamic acid as a biomarker of sarcopenic risk in Japanese people with type 2 diabetes. Nutrients 2023;15:2400. https://www.mdpi.com/2072-6643/15/10/2400/htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moaddel R, Fabbri E, Khadeer MAet al. Plasma biomarkers of poor muscle quality in older men and women from the Baltimore Longitudinal Study of Aging. GERONA 2016;71:1266–72. 10.1093/gerona/glw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, J.P., upon reasonable request.