Abstract
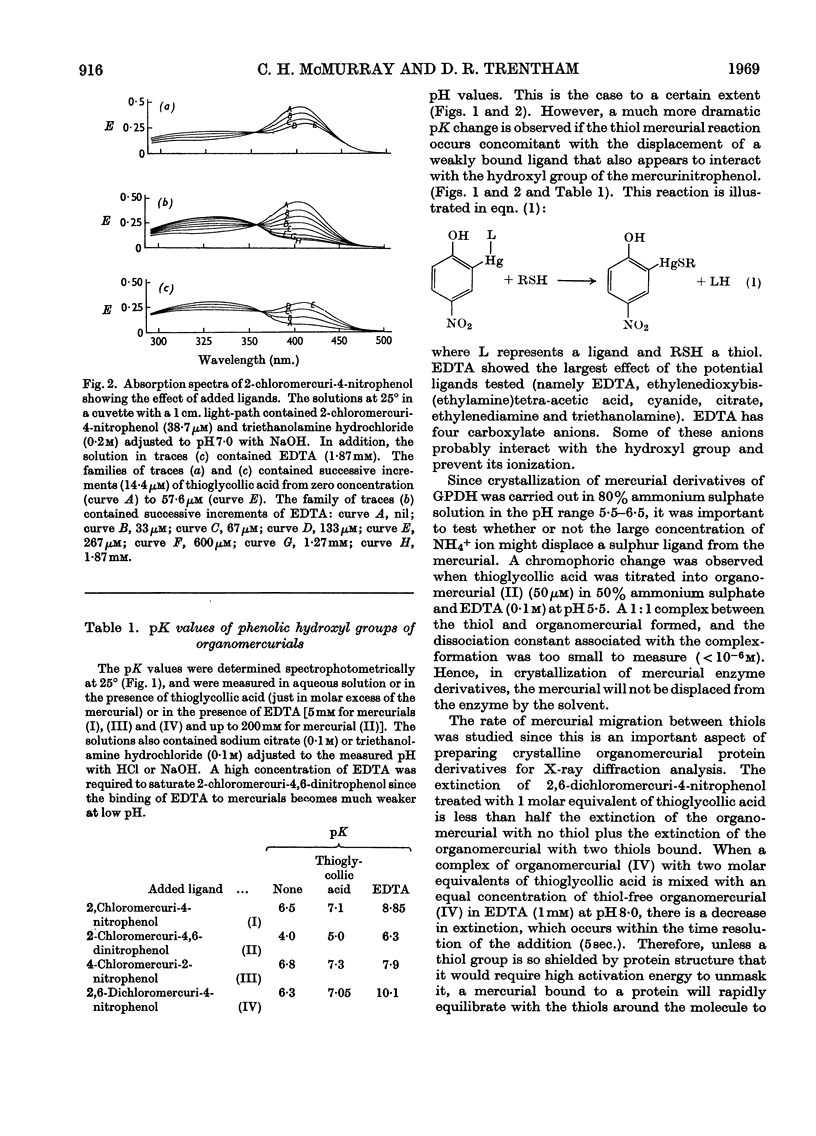
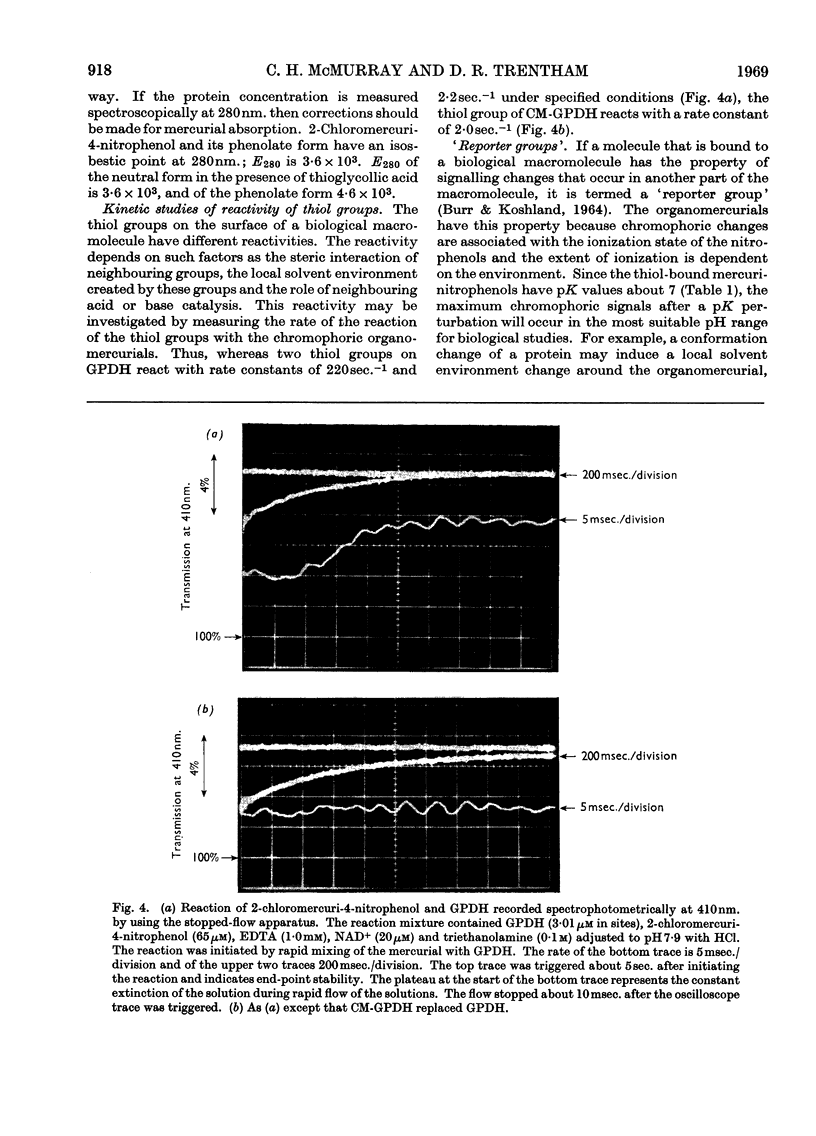
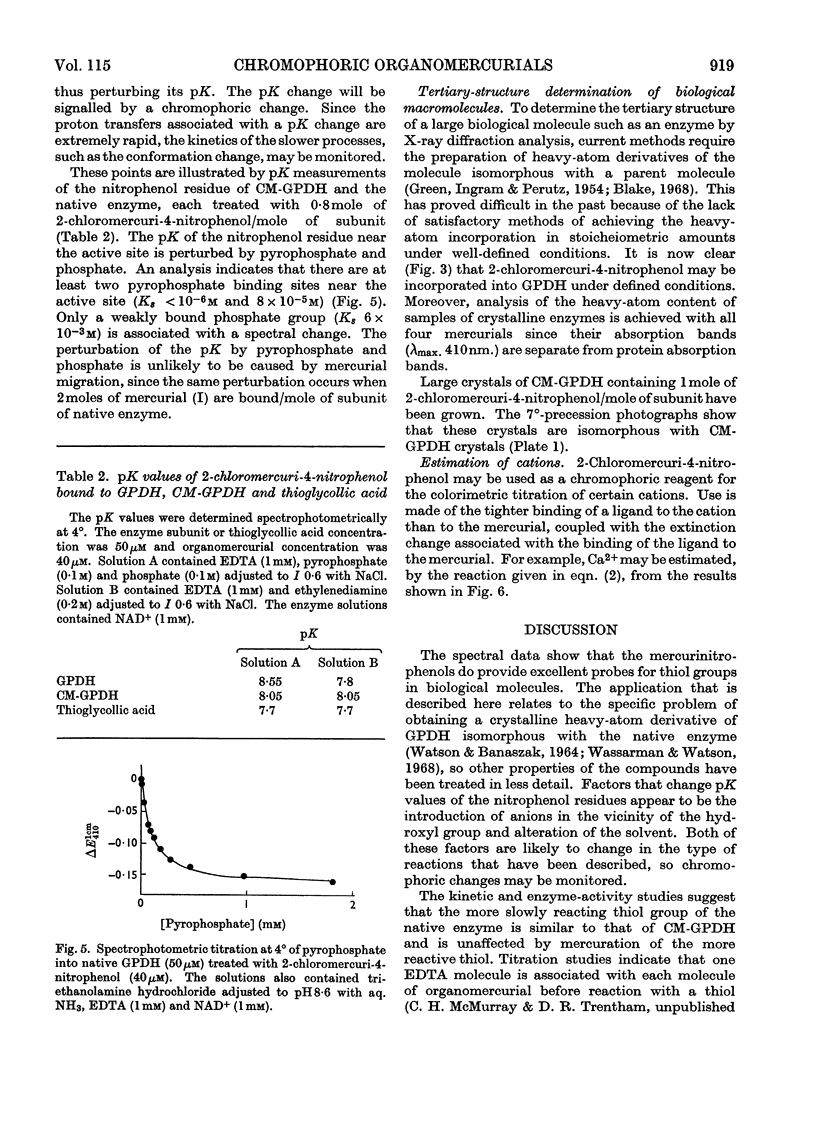
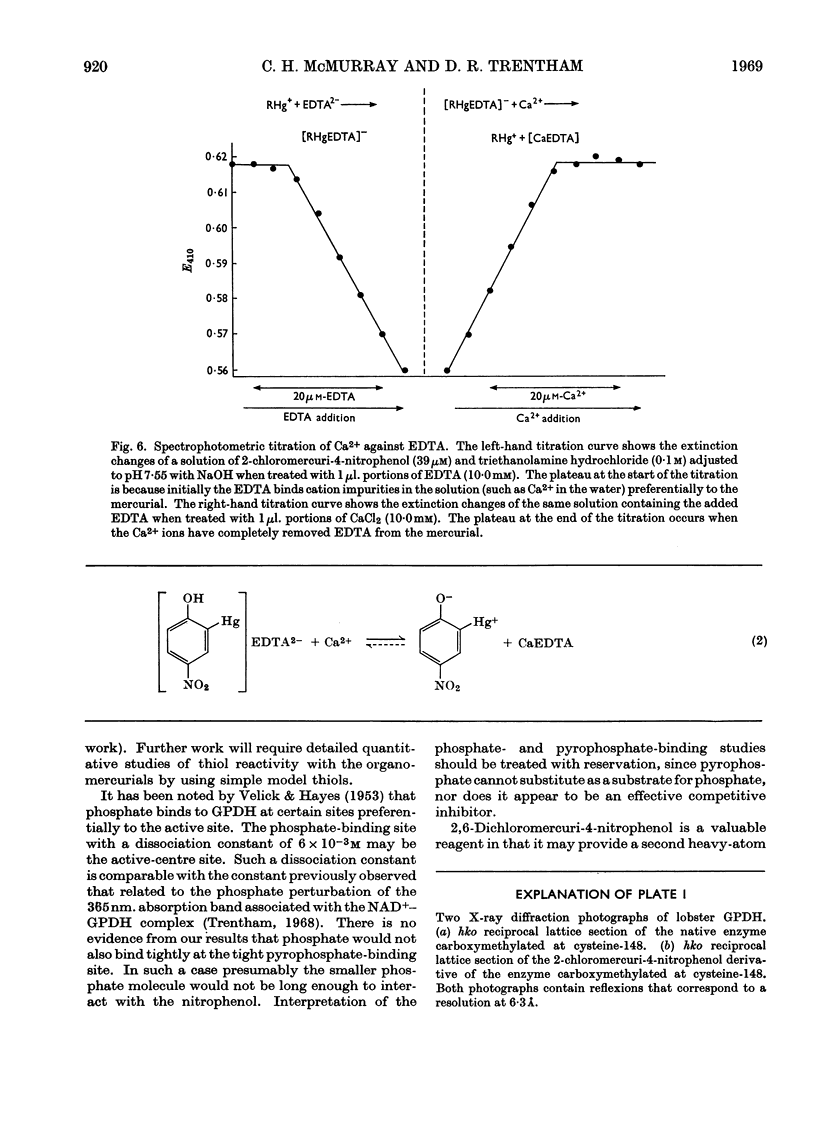
The syntheses of the following organomercurials are described: 2-chloromercuri-4-nitrophenol, 2-chloromercuri-4,6-dinitrophenol, 4-chloromercuri-2-nitrophenol and 2,6-dichloromercuri-4-nitrophenol. All four organomercurials show large spectral changes in the visible spectrum when thiols displace a more weakly bound ligand such as EDTA from the mercury atom. These spectral changes are primarily associated with pK perturbation of the nitrophenols. The mercurials are therefore chromophoric probes for thiol groups in proteins and other thiols of biological interest. The enzyme d-glyceraldehyde 3-phosphate dehydrogenase from lobster muscle is used as a model system in which the properties of the organomercurials may be illustrated. In particular it is shown how d-glyceraldehyde 3-phosphate dehydrogenase carboxymethylated at the active site may be mercurated at a specific site. This mercurial derivative may be crystallized and shown to be isomorphous with the parent enzyme. The mercurials also act as `reporter groups' by monitoring phosphate or pyrophosphate binding to the enzyme. The mercurials may also be used to estimate cations by an EDTA displacement method.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURR M., KOSHLAND D. E., Jr USE OF "REPORTER GROUPS" IN STRUCTURE-FUNCTION STUDIES OF PROTEINS. Proc Natl Acad Sci U S A. 1964 Oct;52:1017–1024. doi: 10.1073/pnas.52.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake C. C. The preparation of isomorphous derivatives. Adv Protein Chem. 1968;23:59–120. doi: 10.1016/s0065-3233(08)60400-3. [DOI] [PubMed] [Google Scholar]
- Davidson B. E., Sajgò M., Noller H. F., Harris J. I. Amino-acid sequence of glyceraldehyde 3-phosphate dehydrogenase from lobster muscle. Nature. 1967 Dec 23;216(5121):1181–1185. doi: 10.1038/2161181a0. [DOI] [PubMed] [Google Scholar]
- HORTON H. R., KOSHLAND D. E., Jr A HIGHLY REACTIVE COLORED REAGENT WITH SELECTIVITY FOR THE TRYPTOPHAN RESIDUE IN PROTEINS. 2-HYDROXY-5-NITROBENZYL BROMIDE. J Am Chem Soc. 1965 Mar 5;87:1126–1132. doi: 10.1021/ja01083a033. [DOI] [PubMed] [Google Scholar]
- Halford S. E., Bennett N. G., Trentham D. R., Gutfeund H. A substate-induced conformation change in the reaction of alkaline phosphatase from Escherichia coli. Biochem J. 1969 Sep;114(2):243–251. doi: 10.1042/bj1140243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R. Aspects of the chemistry of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1968 Oct;109(4):603–612. doi: 10.1042/bj1090603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELICK S. F., HAYES J. E., Jr Phosphate binding and the glyceraldehyde-3-phosphate dehydrogenase reaction. J Biol Chem. 1953 Aug;203(2):545–562. [PubMed] [Google Scholar]
- WATSON H. C., BANASZAK L. J. STRUCTURE OF GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE. STRUCTURE SYMMETRY WITHIN THE MOLECULE. Nature. 1964 Dec 5;204:918–920. doi: 10.1038/204918a0. [DOI] [PubMed] [Google Scholar]