ABSTRACT
RNA-binding protein Nrd1 plays a role in RNA polymerase II transcription termination. In this study, we showed that the orthologous NrdA is important in global mRNA expression and secondary metabolism in Aspergillus species. We constructed an nrdA conditional expression strain using the Tet-On system in Aspergillus luchuenesis mut. kawachii. Downregulation of nrdA caused a severe growth defect, indicating that NrdA is essential for the proliferation of A. kawachii. Parallel RNA-sequencing and RNA immunoprecipitation-sequencing analysis identified potential NrdA-interacting transcripts, corresponding to 32% of the predicted protein-coding genes of A. kawachii. Subsequent gene ontology analysis suggested that overexpression of NrdA affects the production of secondary metabolites. To clarify this, we constructed Aspergillus nidulans, Aspergillus fumigatus, and Aspergillus oryzae strains overexpressing NrdA in the early developmental stage. Overexpression of NrdA reduced the production of sterigmatocystin and penicillin in A. nidulans, as well as that of helvolic acid and pyripyropene A in A. fumigatus. Moreover, it increased the production of kojic acid and reduced the production of penicillin in A. oryzae. These effects were accompanied by almost consistent changes in the mRNA levels of relevant genes. Collectively, these results suggest that NrdA is the essential RNA-binding protein, which plays a significant role in global gene expression and secondary metabolism in Aspergillus species.
IMPORTANCE
Nrd1, a component of the Nrd1–Nab3–Sen1 complex, is an essential RNA-binding protein involved in transcriptional termination in yeast. However, its role in filamentous fungi has not been studied. In this study, we characterized an orthologous NrdA in the Aspergillus species, identified potential NrdA-interacting mRNA, and investigated the effect of overexpression of NrdA on mRNA expression in Aspergillus luchuensis mut. kawachii. The results indicated that NrdA controls global gene expression involved in versatile metabolic pathways, including the secondary metabolic process, at least in the early developmental stage. We demonstrated that NrdA overexpression significantly affected the production of secondary metabolites in Aspergillus nidulans, Aspergillus oryzae, and Aspergillus fumigatus. Our findings are of importance to the fungal research community because the secondary metabolism is an industrially and clinically important aspect for the Aspergillus species.
KEYWORDS: Aspergillus, RNA-binding protein, NrdA, gene expression, secondary metabolism
INTRODUCTION
There are at least two pathways for transcription termination in the budding yeast Saccharomyces cerevisiae, namely, the RNA polymerase II cleavage/polyadenylation factor-related pathway and the Nrd1–Nab3–Sen1 (NNS)-related pathway (1, 2). The former cleavage/polyadenylation factor pathway is required for polyadenylation of the 3′ end of precursor mRNA. The latter pathway is required for the early termination of transcripts to produce shorter polyadenylated transcripts. Moreover, it is involved in the subsequent production of non-coding transcripts, such as small nucleolar RNAs, small nuclear RNAs, cryptic unstable transcripts, and stable uncharacterized transcripts with largely unknown functions. The Nrd1 and Nab3 are essential RNA-binding proteins and bind specific sequences in target RNA, whereas Sen1 is a helicase responsible for the termination of transcription. The NNS complex has been extensively studied from the viewpoint of non-coding RNA production. However, it has been recently suggested that the NNS-related pathway also plays a significant role in protein-coding mRNA involved in response to nutrient starvation (3, 4). It was proposed that the binding of Nrd1 and Nab3 led to premature transcription termination, resulting in the downregulation of mRNA levels. In S. cerevisiae, it was estimated that Nrd1 and Nab3 directly regulate a variety of protein-coding genes, representing 20%–30% of protein-coding transcripts (5). In addition, the Nrd1 ortholog Seb1 drives transcription termination for both protein-coding and non-coding sequences in the fission yeast Schizosaccharomyces pombe (6, 7).
We have investigated the mechanism of citric acid accumulation by the white koji fungus Aspergillus luchuensis mut. kawachii, which is used for the production of shochu, a Japanese traditional distilled spirit. A. kawachii is used as a producer of starch-degrading enzymes, such as α-amylase and glucoamylase (8, 9). In addition, A. kawachii also produces a large amount of citric acid, which prevents the growth of microbial contaminants during the fermentation process (10). During the study, we found that the Nrd1 ortholog encoding nrdA was located in a syntenic region together with mitochondrial citrate synthase-encoding citA and mitochondrial citrate transporter encoding yhmA in the subdivision Pezizomycotina (including genus Aspergillus). However, it was not found in the subdivisions Saccharomycotina (including genus Saccharomyces) and Taphrinomycotina (including genus Schizosaccharomyces) (11). Considering the numerous examples of metabolic gene clusters in fungi and plants (12, 13), this finding motivated us to study the function of NrdA in Aspergillus species in terms of metabolic activity.
In this study, we showed that NrdA is an essential RNA-binding protein involved in the production of citric acid in A. kawachii using the nrdA conditional expression strain. Nevertheless, whether nrdA is functionally related to citA and yhmA remains unclear. By combining RNA-sequencing (RNA-seq) and RNA immunoprecipitation-sequencing (RIP-seq) analyses, we identified potential NrdA–mRNA interactions, corresponding to approximately 32% of protein-coding genes of the A. kawachii genome. In addition, we showed that overexpression of nrdA causes significant changes in the expression levels of NrdA-interacting mRNA involved in secondary metabolism. Furthermore, we demonstrated that overexpression of nrdA also caused changes in the production of secondary metabolites in Aspergillus nidulans, Aspergillus fumigatus, and Aspergillus oryzae. These results suggested that the NrdA-associated transcription termination pathway potentially regulates the secondary metabolic process in the Aspergillus species.
RESULTS
NrdA orthologs are conserved in Aspergillus species
The A. kawachii nrdA gene encodes a protein composed of 742 amino acid residues. BLASTP analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi) showed that the amino acid sequence percent identities between A. kawachii NrdA and S. cerevisiae Nrd1, and between A. kawachii NrdA and Schiz. pombe Seb1 were 48.57% and 34.52%, respectively. The RNA polymerase II C-terminal domain-interacting domain (CID) (1–150 amino acid residues of S. cerevisiae Nrd1) (14–16) and RNA recognition motif (RRM) (339–407 amino acid residues of S. cerevisiae Nrd1) (16, 17) are well conserved in A. kawachii NrdA (Fig. S1). The Pfam domain analysis (https://pfam.xfam.org/) also confirmed the presence of CID and RRM in the relevant regions of A. kawachii NrdA (data not shown). On the other hand, the amino acid residues of the Nab3-binding domain, arginine-glutamate/arginine-serine-rich domain, and the C-terminal proline/glutamine (P/Q)-rich low-complexity domain (LCD) (151–214, 245–265, and 513–575 amino acid residues of S. cerevisiae Nrd1, respectively) (16, 18, 19) were less conserved in the A. kawachii NrdA. The Nab3-binding domain, arginine-glutamate/arginine-serine-rich domain, and P/Q-rich LCD of A. kawachii NrdA were more similar to those of Schiz. pombe Seb1 than those of S. cerevisiae Nrd1. In addition, amino acid residues between RRM and P/Q rich LCD were well conserved among S. cerevisiae Nrd1, Schiz. pombe Seb1, and A. kawachii NrdA. Phylogenetic analysis supported that Nrd1 orthologs of subdivision Pezizomycotina (including A. kawachii NrdA) are closer to those of subdivision Taphrinomycotina (including Schiz. pombe Seb1) than those of subdivision Saccharomycotina (including S. cerevisiae Nrd1) (Fig. S2).
nrdA is an essential gene in A. kawachii
To investigate the physiologic role of nrdA, we attempted to construct A. kawachii nrdA disruptant. However, all of the transformants obtained by the nrdA disruption cassette were heterokaryotic gene disruptants (see Fig. S3; Table S1 in the supplemental material). Therefore, we constructed a Tet-nrdA strain that conditionally expressed nrdA under the control of a Tet-On promoter. The Tet-nrdA strain exhibited significantly deficient growth in yeast extract sucrose (YES) agar medium without doxycycline (Dox) (nrdA depletion condition) (Fig. 1A), indicating that disruption of nrdA induces lethality.
Fig 1.
(A) Colony formation of A. kawachii control and Tet-nrdA strains. Conidia (104) were inoculated onto YES medium. Strains were cultured at 30°C for 5 days on YES medium with or without doxycycline (Dox). Conidia of each strain (1 × 104) were inoculated onto agar medium. (B) Citric acid production of control and Tet-nrdA strains. Strains were precultured in YES medium with 1 µg/mL Dox for 16 h, transferred to CAP medium, and further cultivated for 48 h. The mean and standard deviation were determined from the results of 3 independent cultivations. *, statistically significant difference (P < 0.05, Welch’s t-test) relative to the data obtained for the control strain.
Gene expression level of nrdA affects citric acid productivity
Next, we tested the effect of NrdA expression on citric acid production because nrdA was located in the syntenic region with mitochondrial citrate synthase encoding citA and citrate transporter encoding yhmA (11). We compared the production of citric acid by A. kawachii control and Tet-nrdA strains (Fig. 1B). The control strain was precultivated in YES medium at 30°C for 16 h, transferred to citric acid production (CAP) medium (an optimized medium for CAP) (11), and further cultured at 30°C for 48 h. Because the Tet-nrdA strain showed severe growth defect in the absence of Dox, it was precultivated in the YES medium with Dox and transferred to the CAP medium with or without Dox. Following cultivation, the concentration of citric acid in the culture supernatant and mycelial biomass was measured to determine the extracellular CAP per mycelial weight. Based on the level of citric acid in the culture supernatant and the amount of mycelial biomass produced, the Tet-nrdA strain cultivated without Dox showed similar CAP compared with the control strain. By contrast, the Tet-nrdA strain cultivated with Dox exhibited only approximately 9% of the CAP of the control strain. These results suggest that the expression level of NrdA significantly influences CAP in A. kawachii. Combined with the results described below, the overexpression of nrdA driven by the Tet-On promoter may inhibit CAP.
NrdA is localized in the nucleus
To analyze the subcellular localization of NrdA in A. kawachii, NrdA tagged with a green fluorescent protein (GFP-NrdA) and histone H2B tagged with a monomeric red fluorescent protein (mRFP) (H2B-mRFP, a nuclear marker protein) were co-expressed in the Tet-nrdA strain. The GFP-NrdA was expressed by the native nrdA promoter. Functional expression of GFP-NrdA was confirmed by the viable phenotype of the Tet-nrdA plus GFP-nrdA plus H2B-mRFP strain in YES agar medium without Dox (Fig. 2A). The Tet-nrdA plus GFP-nrdA plus H2B-mRFP strain was cultivated in minimal liquid medium for 16 h without shaking and observed by fluorescence microscopy. Green fluorescence associated with GFP-NrdA merged with the red fluorescence of H2B-mRFP (Fig. 2B), indicating that the GFP-NrdA localizes in the nucleus. In addition, speckles of green fluorescence were observed in the nucleus. It was reported that the Nrd1-dependent nuclear speckles appear in S. cerevisiae under glucose starvation conditions (3). Thus, it might be possible that NrdA also forms these speckles in A. kawachii in response to some environmental stress.
Fig 2.
(A) Colony formation of A. kawachii Tet-nrdA strain and its complemented strain with GFP-NrdA and H2B-mRFP. Conidia (1 × 104) were inoculated onto YES agar medium with or without 1 µg/mL Dox and incubated at 30°C for 5 days. (B) Fluorescence microscopic observation of the GFP-NrdA- and H2B-mRFP-expressing strain. Scale bars indicate 10 µm.
Examination of experimental conditions for transcriptome analysis
We performed transcriptome analysis to investigate the NrdA-associated transcripts. For this purpose, we constructed a Tet-S-nrdA strain, which expresses S-tagged NrdA (S-NrdA) under the control of the Tet-On promoter. The Tet-S-nrdA strain formed colonies in the YES agar medium with Dox, whereas it showed severe growth defect in the YES agar medium without Dox. These findings confirmed that the Tet-On system regulates the functional S-NrdA (Fig. 3A). In addition, the expression of S-NrdA was confirmed by the detection of a band of the predicted size of the S-NrdA protein through S-protein affinity purification (Fig. 3B left panel) and detection by immunoblotting using an anti-S-tag antibody (Fig. 3B right panel).
Fig 3.
(A) Colony formation of A. kawachii control and Tet-S-nrdA strains. Strains were cultured at 30°C for 5 days on YES medium with or without Dox. Conidia of each strain (1 × 104) were inoculated onto agar medium. (B) Immunoblotting analysis of purified S-NrdA protein from the A. kawachii Tet-S-nrdA strain. The apparent molecular mass of S-NrdA was 80.7 kDa. (C) Scheme of experimental design for the RNA-seq and RIP-seq analyses to identify the NrdA-interacting transcripts and investigate the effect of NrdA overexpression. (D) Quantitative RT-PCR analysis to evaluate the expression levels of nrdA and S-nrdA in A. kawachii control and Tet-S-nrdA strains. (E) Cluster analysis to confirm the S-nrdA expression condition in the A. kawachii Tet-S-nrdA strain in the presence or absence of Dox. The mean and standard deviation were determined from the results of 3 independent cultivations. *, statistically significant difference (P < 0.05, Welch’s t-test) relative to the data obtained for the control strain.
For the identification of NrdA–mRNA interaction and evaluation of the destination of mRNA interacted with NrdA, we performed an experiment under the following conditions. The Tet-S-nrdA strain was precultivated in YES medium with Dox; next, mycelia were transferred to YES medium with or without Dox and further cultivated (Fig. 3C). The resultant mycelial cells cultivated with Dox were separated into two portions and used for RNA-seq analysis and RIP-seq analysis to identify the NrdA–mRNA interaction by comparing their RNA pools. In addition, the resultant mycelial cells cultivated without Dox were used for RNA-seq analysis to investigate the expression levels of NrdA-interacting mRNA in the presence or absence of Dox.
For the latter purpose, we evaluated the expression level of S-nrdA in the Tet-S-nrdA strain cultivated in the presence or absence of Dox. The Tet-S-nrdA strain was precultivated in YES medium with Dox for 16 h; next, mycelia were transferred to YES medium with or without Dox and further cultivated for 4, 6, 12, or 24 h. In addition, the A. kawachii control strain was precultivated in the YES medium for 16 h; subsequently, the mycelia were transferred to YES medium and further cultivated for 24 h. After the cultivations, total RNA was extracted and used to compare the expression levels of S-nrdA (Fig. 3D). The levels of S-nrdA in the Tet-S-nrdA strain cultivated in the medium with Dox were significantly higher (17.3-fold to 37.2-fold higher) than those of nrdA in the control strain, indicating that S-NrdA was overexpressed throughout the cultivation period. On the other hand, the expression level of S-nrdA in the Tet-S-nrdA strain cultivated in the medium without Dox was gradually decreased; however, it remained comparable to the expression level of nrdA in the control strain after cultivation for 12 h and 24 h. This result indicated that the S-nrdA transcript was not depleted even after cultivation without Dox for 24 h. Thus, we considered the cultivation of the Tet-S-nrdA strain with and without Dox as S-nrdA overexpression and expression condition, respectively. To confirm this consideration, we additionally performed RNA-seq analysis of the control strain cultivated in YES medium without Dox (Fig. 3C, an experimental condition was surrounded by the dotted line), followed by clustering analysis of RNA-seq along with the RNA-seq and RIP-seq data obtained using the Tet-S-nrdA strain (Fig. 3E, see Fig. S4 in the supplemental material). The results indicated that the transcriptomic profile of the control strain is more similar to that of the Tet-S-nrdA strain cultivated without Dox than that of the Tet-S-nrdA strain cultivated with Dox. In addition, this is consistent with the data showing that the Tet-nrdA strain cultivated without Dox showed similar CAP compared with the control strain. By contrast, the Tet-nrdA strain cultivated with Dox exhibited significantly reduced CAP compared with the control strain (Fig. 1B).
NrdA interacts with 32% of mRNA in A. kawachii
Based on the examination of experimental conditions, we cultivated the Tet-S-nrdA strain in YES medium with or without Dox as S-nrdA overexpression and expression condition, respectively, and the mycelia were subjected to RNA-seq and RIP-seq (Fig. 3C). A 4.4-fold higher amount of RNA was obtained from the mycelia of the Tet-S-nrdA strain cultivated with Dox by RIP using the anti-S-tag antibody compared with that obtained using the normal rabbit IgG (negative control) (Fig. 4A). This result indicated that the enrichment of NrdA-associated RNA was successful. To identify the NrdA–mRNA interaction, we compared the mRNA profiles of the Tet-S-nrdA strain cultivated with Dox obtained from the RNA-seq and RIP-seq analyses (Fig. 3C). We predicted NrdA–mRNA interaction by identifying the mRNA significantly enriched by RIP based on the following criteria: log2 fold change >0 and q-value <0.05 (see Data Set S1 in the supplemental material). This analysis identified 3,676 mRNA transcripts that represent 32% of the total 11,474 predicted coding sequences of A. kawachii.
Fig 4.
(A) The RNA obtained by immunoprecipitation from the Tet-S-nrdA strain cultivated in the presence of Dox. Anti-S-tag antibody and normal rabbit IgG (as a negative control) were used for the RNA immunoprecipitation. The obtained RNA was measured by NanoDrop-8000 (Thermo Fisher Scientific). The mean and standard deviation were determined from the results of 3 independent cultivations. *, a statistically significant difference (P < 0.05, Welch’s t-test) relative to the negative control. (B) Change in gene expression of putative NrdA-interacting transcripts by the overexpression of NrdA. Predicted NrdA-interacting transcripts were plotted according to the log2 fold enrichment detected by the RIP analysis on the X-axis and the log2 fold change in gene expression observed after the overexpression of NrdA on the Y-axis. All the plotted data showed statistical significance (q < 0.05).
We performed gene ontology (GO) enrichment analysis of mRNA transcripts, which were predicted to interact with NrdA. The results of this analysis identified metabolic process-, transport process-, and transcriptional regulation-related terms (Table 1). In addition, enriched terms included numerous secondary metabolic-related terms, such as the secondary metabolic process (GO:0019748) and mycotoxin biosynthetic process (GO:0043386).
TABLE 1.
Biological process GO terms of potential NrdA-interacting mRNA identified by AspGD GO Term Finder
| GO ID | GO term | Number in gene set | Number in background | P-value |
|---|---|---|---|---|
| GO:0019748 | Secondary metabolic process | 160 | 310 | 1.23E−27 |
| GO:0055085 | Transmembrane transport | 307 | 799 | 2.68E−24 |
| GO:0055114 | Oxidation–reduction process | 339 | 984 | 1.49E−17 |
| GO:0044550 | Secondary metabolite biosynthetic process | 115 | 243 | 2.21E−15 |
| GO:0008152 | Metabolic process | 1226 | 4844 | 7.69E−09 |
| GO:0043386 | Mycotoxin biosynthetic process | 56 | 113 | 1.80E−07 |
| GO:0043385 | Mycotoxin metabolic process | 57 | 116 | 1.88E−07 |
| GO:0009404 | Toxin metabolic process | 58 | 119 | 1.95E−07 |
| GO:0018958 | Phenol-containing compound metabolic process | 43 | 77 | 2.01E−07 |
| GO:0009403 | Toxin biosynthetic process | 56 | 115 | 4.28E−07 |
| GO:0051234 | Establishment of localization | 378 | 1315 | 1.68E−06 |
| GO:0042537 | Benzene-containing compound metabolic process | 31 | 50 | 2.45E−06 |
| GO:0018130 | Heterocycle biosynthetic process | 237 | 766 | 3.76E−06 |
| GO:1901362 | Organic cyclic compound biosynthetic process | 253 | 828 | 3.86E−06 |
| GO:0046189 | Phenol-containing compound biosynthetic process | 35 | 61 | 4.15E−06 |
| GO:0006810 | Transport | 369 | 1289 | 4.73E−06 |
| GO:1901376 | Organic heteropentacyclic compound metabolic process | 44 | 86 | 5.18E−06 |
| GO:1901378 | Organic heteropentacyclic compound biosynthetic process | 43 | 84 | 7.69E−06 |
| GO:0051179 | Localization | 394 | 1402 | 1.47E−05 |
| GO:1903506 | Regulation of nucleic acid-templated transcription | 216 | 697 | 1.76E−05 |
| GO:0006355 | Regulation of transcription, DNA-templated | 216 | 697 | 1.76E−05 |
| GO:2001141 | Regulation of RNA biosynthetic process | 216 | 698 | 2.01E−05 |
| GO:1900813 | Monodictyphenone metabolic process | 23 | 34 | 3.07E−05 |
| GO:1900815 | Monodictyphenone biosynthetic process | 23 | 34 | 3.07E−05 |
| GO:0051252 | Regulation of RNA metabolic process | 219 | 714 | 3.57E−05 |
| GO:1900555 | Emericellamide metabolic process | 22 | 32 | 4.03E−05 |
| GO:1900557 | Emericellamide biosynthetic process | 22 | 32 | 4.03E−05 |
| GO:0050761 | Depsipeptide metabolic process | 22 | 32 | 4.03E−05 |
| GO:0050763 | Depsipeptide biosynthetic process | 22 | 32 | 4.03E−05 |
| GO:1901334 | Lactone metabolic process | 24 | 37 | 5.21E−05 |
| GO:1901336 | Lactone biosynthetic process | 24 | 37 | 5.21E−05 |
| GO:0042180 | Cellular ketone metabolic process | 44 | 94 | 0.00016 |
| GO:0072330 | Monocarboxylic acid biosynthetic process | 50 | 113 | 0.0002 |
| GO:1901503 | Ether biosynthetic process | 22 | 34 | 0.00021 |
| GO:0030638 | Polyketide metabolic process | 22 | 34 | 0.00021 |
| GO:0030639 | Polyketide biosynthetic process | 21 | 32 | 0.00029 |
| GO:0005975 | Carbohydrate metabolic process | 138 | 422 | 0.00032 |
| GO:0042181 | Ketone biosynthetic process | 39 | 81 | 0.00034 |
| GO:1900582 | -Orsellinic acid metabolic process | 16 | 21 | 0.00038 |
| GO:1900584 | -Orsellinic acid biosynthetic process | 16 | 21 | 0.00038 |
| GO:1900552 | Asperfuranone metabolic process | 20 | 30 | 0.00039 |
| GO:1900554 | Asperfuranone biosynthetic process | 20 | 30 | 0.00039 |
| GO:1902644 | Tertiary alcohol metabolic process | 20 | 30 | 0.00039 |
| GO:1902645 | Tertiary alcohol biosynthetic process | 20 | 30 | 0.00039 |
| GO:0045460 | Sterigmatocystin metabolic process | 36 | 73 | 0.00047 |
| GO:2000112 | Regulation of cellular macromolecule biosynthetic process | 229 | 775 | 0.00048 |
| GO:0010556 | Regulation of macromolecule biosynthetic process | 230 | 779 | 0.00049 |
| GO:0031326 | Regulation of cellular biosynthetic process | 238 | 813 | 0.00063 |
| GO:0019438 | Aromatic compound biosynthetic process | 211 | 707 | 0.00066 |
| GO:0045461 | Sterigmatocystin biosynthetic process | 23 | 71 | 0.00071 |
| GO:0009889 | Regulation of biosynthetic process | 240 | 822 | 0.00071 |
| GO:0034311 | Diol metabolic process | 25 | 44 | 0.0011 |
| GO:0034312 | Diol biosynthetic process | 25 | 44 | 0.0011 |
| GO:0019219 | Regulation of nucleobase-containing compound metabolic process | 222 | 755 | 0.00112 |
| GO:1901615 | Organic hydroxy compound metabolic process | 91 | 258 | 0.00116 |
| GO:1901617 | Organic hydroxy compound biosynthetic process | 62 | 158 | 0.00127 |
| GO:0010468 | Regulation of gene expression | 232 | 797 | 0.00145 |
| GO:0006351 | Transcription, DNA-templated | 124 | 384 | 0.00284 |
| GO:0097659 | Nucleic acid-templated transcription | 124 | 384 | 0.00284 |
| GO:0009820 | Alkaloid metabolic process | 26 | 49 | 0.00387 |
| GO:0009821 | Alkaloid biosynthetic process | 26 | 49 | 0.00387 |
| GO:0032774 | RNA biosynthetic process | 124 | 387 | 0.00443 |
| GO:0080090 | Regulation of primary metabolic process | 253 | 899 | 0.00828 |
| GO:0018904 | Ether metabolic process | 23 | 43 | 0.01271 |
| GO:0060255 | Regulation of macromolecule metabolic process | 256 | 919 | 0.01666 |
| GO:0035834 | Indole alkaloid metabolic process | 23 | 45 | 0.03425 |
| GO:0035835 | Indole alkaloid biosynthetic process | 23 | 45 | 0.03425 |
NrdA overexpression downregulates transcripts with high levels of NrdA interaction
Next, to examine the significance of NrdA–mRNA interaction, differential gene expression between the S-NrdA expression and overexpression conditions was compared using RNA-seq (see Data Set S1 in the supplemental material). A q-value <0.05 denoted statistically significant changes in gene expression. This criterion was set to prevent the loss of upregulation or downregulation of the potential NrdA-interacting transcripts. Based on this criterion, the predicted 3,676 NrdA-associated mRNAs included 1,768 downregulated genes and 674 upregulated genes by the overexpression of S-NrdA. These findings indicated that approximately half of NrdA-associated mRNAs were downregulated by the overexpression of S-NrdA. In addition, RIP-seq and RNA-seq plotting analyses showed that the proportion of downregulated genes was increased in the higher enriched mRNA transcript by RIP (predicted strong interaction of mRNA with S-NrdA) (Fig. 4B). The highly enriched mRNAs showed a larger proportion of those that are downregulated by overexpression of nrdA. For example, the higher enriched mRNA transcript (with log2 fold change >5) included 10 genes downregulated by the overexpression of S-NrdA (surrounded by the dotted line in Fig. 4B). This trend was found to be proportional to the ratio of enrichment (confirmed with different log2 fold changes > 0, 1, 2, 3, 4, or 5) (see Fig. S5 in the supplemental material). Based on the information obtained from domain search using the Pfam database, the 10 higher enriched mRNA transcripts encode have beta-lactamase (AKAW_10564), G-protein-coupled receptor (AKAW_10304), aldehyde reductase (AKAW_01947), or tannanase (AKAW_10686 and AKAW_04699) functions; however, most of them are hypothetical proteins with unknown function (Table S2). In addition, S. cerevisiae and Schiz. pombe have homologs only for AKAW_01947 among these 10 genes.
NrdA affects secondary metabolism in three Aspergillus species
GO analysis indicated that the significant change observed in mRNA levels of secondary metabolic genes affected by NrdA in A. kawachii (Table 1). Specifically, four NRPS (non-ribosomal peptide synthetase) genes (AKAW_01949, AKAW_05409, AKAW_07855, and AKAW_11089), five polyketide synthase (PKS) genes (AKAW_06360, AKAW_06695, AKAW_07291, AKAW_07931, and AKAW_08325), and a PKS-NRPS hybrid enzyme gene (AKAW_10071) were included, and all of these genes were downregulated by the overexpression of NrdA (see Data Set S1 in the supplemental material). This result was interesting because the relationships between the NNS complex pathway and the production of secondary metabolites have not been studied. However, the secondary metabolites of A. kawachii are currently largely unknown. Therefore, we tested the effect of overexpression of intrinsic NrdA on the production of secondary metabolites in A. nidulans, A. fumigatus, and A. oryzae.
A. nidulans OE-nrdA strain showed a slightly fluffy phenotype (Fig. 5A) with a reduced number of conidia (Fig. 5B). To investigate the production of penicillin and sterigmatocystin, A. nidulans strains were precultivated in YES medium at 30°C for 16 h, transferred to minimal medium, and further cultivated at 30°C for 24, 48, or 72 h. The results of the halo assay indicated that the OE-nrdA strain showed significantly reduced penicillin production compared with the control strain (Fig. 5C). The sizes of the halos were significantly different using culture supernatant obtained after 24 and 48 h, whereas they were similar at 72 h. This evidence indicates that overexpression of NrdA suppressed the production of penicillin in the early cultivation period. In addition, the production of sterigmatocystin by OE-nrdA was 26% of that produced by the control strain after 24 h of cultivation (Fig. 5D, left side). This difference was reduced after 48 h of cultivation (Fig. 5D, right side). We confirmed that mRNA levels of nrdA in OE-nrdA were 15-fold higher compared with those detected in the control strain at 12 h (Fig. 5E). The differences in the mRNA levels of nrdA in OE-nrdA were not statistically significant at 24 and 48 h, indicating that nrdA is overexpressed in the early cultivation period of the OE-nrdA strain. Next, we investigated whether the reduced production of penicillin and sterigmatocystin was controlled at the mRNA level. The mRNA levels of ipnA (an isopenicillin-N synthase with a role in penicillin biosynthesis) (20), aflR (a transcriptional regulator involved in the production of sterigmatocystin) (21), and stcU (a putative versicolorin reductase involved in the biosynthesis of sterigmatocystin) (22, 23) were significantly reduced in the OE-nrdA strain at 12 and 24 h compared with the control strain. However, these levels became similar at 48 h. This is consistent with the data showing that the higher mRNA level of nrdA and reduced productions of penicillin and sterigmatocystin were significant in the early cultivation period.
Fig 5.
(A) Colony formation of the A. nidulans control and OE-nrdA strains. Conidia (1 × 104) were inoculated onto a minimal agar medium with biotin and cultured at 30°C for 7 days. (B) Conidiation of the control and OE-nrdA strains. (C) Penicillin bioassay of the A. nidulans control and OE-nrdA strains. (D) Production of sterigmatocystin by the control and OE-nrdA strains. (E) mRNA levels of nrdA, ipnA, aflR, and stcU. The control and OE-nrdA strains were precultured in YES medium for 16 h, transferred to a minimal medium with biotin, and further cultured for 12, 24, and 48 h. The mean and standard deviation were determined from the results of 3 independent cultivations. Asterisks indicate significant differences (*P < 0.05, Welch’s t-test) versus the results obtained for the control strain.
The A. fumigatus OE-nrdA strain showed similar colony sizes (Fig. 6A) with reduced number of conidia (Fig. 6B). Next, we analyzed the levels of fumagillin, helvolic acid, and pyripyropene A in culture supernatant (Fig. 6C). A. fumigatus strains were cultivated in minimal liquid medium with fetal bovine serum (FBS) at 30°C for 24 h. The difference in the production of fumagillin between the control and OE-nrdA strains was not statistically significant. By contrast, the production of helvolic acid and pyripyropene by OE-nrdA was significantly reduced to 47% and 3% of that observed in the control strain, respectively. We confirmed that the OE-nrdA strain exhibited higher expression of nrdA compared with the control strain (Fig. 6D). Subsequently, we investigated the gene expression of fumR (a putative C6 type transcription factor-encoding gene involved in fumagillin biosynthesis) (24), helA (an oxidosqualene cyclase-encoding gene involved in the production of helvolic acid) (25), and pyr2 (a PKS-encoding gene involved in the biosynthesis of pyripyropene A) (26). Although the mRNA levels of fumR in the control and OE-nrdA strains were similar, those of helA and pyr2 were lower in the OE-nrdA strain (Fig. 6D). This was consistent with the results demonstrating that the production of helvolic acid and pyripyropene A was significantly suppressed by the overexpression of NrdA.
Fig 6.
(A) Colony formation of the A. fumigatus control and OE-nrdA strains. Conidia (1 × 104) were inoculated onto minimal agar medium and cultured at 30°C or 42°C for 5 days. (B) Conidiation of the control and OE-nrdA strains. (C) Production of fumagillin, helvolic acid, and pyripyropene A by the control and OE-nrdA strains. Conidia (2 × 107 cells) were inoculated in a minimal liquid medium with 5% FBS and cultivated with shaking (163 rpm) at 30°C for 24 h. (D) mRNA levels of nrdA, fumR, helA, and pyr2. The strains were cultivated in a minimal liquid medium with 5% FBS for 12 h. The mean and standard deviation were determined from the results of 3 independent cultivations. Asterisks indicate significant differences (*P < 0.05, Welch’s t-test) versus the results obtained for the control strain.
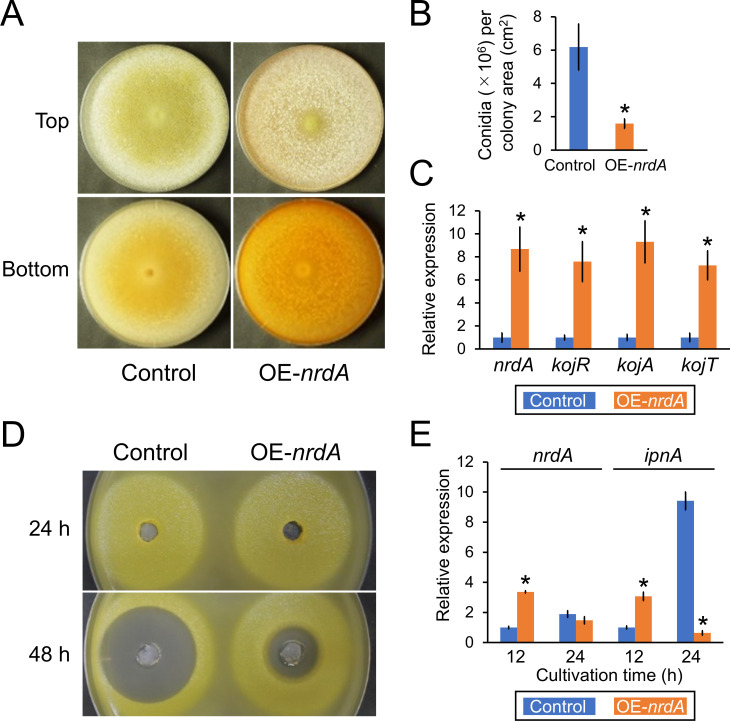
A. oryzae control and OE-nrdA strains were cultivated in a medium containing ferric ion (27), whose color turns red if it is chelated by kojic acid (Fig. 7A). The OE-nrdA strain showed a reduced number of conidia (Fig. 7B). In addition, the number of sclerotia increased in the OE-nrdA strain compared with the control strain (Fig. 7A). The bottom side of the colony of the OE-nrdA strain exhibited a red color, indicating that this strain produces higher amounts of kojic acid compared with the control strain. To investigate the mRNA levels of genes involved in the production of kojic acid, the strains were cultivated in a kojic acid production liquid medium at 30°C for 5 days. Furthermore, we confirmed a higher mRNA level of nrdA in the OE-nrdA strain compared with that detected in the control strain (Fig. 7C). In addition, the gene expression of kojR, kojA, and kojT, which are involved in the production of kojic acid (27), also exhibited higher levels compared with those measured in the control strain. To investigate the production of penicillin, strains were precultured in YES medium, transferred to a minimal medium, and further cultivated for 24 or 48 h. The results of the halo assay using the culture supernatant at 48 h revealed significantly reduced production of penicillin by the OE-nrdA strain (Fig. 7D). In addition, the analysis confirmed that the OE-nrdA strain showed higher and similar mRNA levels of nrdA compared with the control strain at 12 and 24 h, respectively (Fig. 7E). In addition, the OE-nrdA strain showed higher and lower mRNA levels of ipnA compared with the control strain at 12 and 24 h, respectively. The significantly lower mRNA level of ipnA is consistent with the data indicating that penicillin production was suppressed by the overexpression of nrdA at 48 h.
Fig 7.
(A) Colony formation of the A. oryzae control and OE-nrdA strains. Conidia (1 × 104) were inoculated onto kojic acid production agar medium and cultured at 30°C for 5 days. (B) Conidiation of the control and OE-nrdA strains. (C) mRNA levels of nrdA, kojR, kojA, and kojT. Conidia (2 × 107 cells) were inoculated in kojic acid production liquid medium and cultivated with shaking (163 rpm) at 30°C for 5 days. (D) Penicillin bioassay of the A. oryzae control and OE-nrdA strains. (E) mRNA levels of nrdA and ipnA. The mean and standard deviation were determined from the results of 3 independent cultivations. Asterisks indicate significant differences (*P < 0.05, Welch’s t-test) versus the results obtained for the control strain.
DISCUSSION
The NNS-related transcriptional termination pathway has been extensively characterized in yeast S. cerevisiae and Schiz. pombe (1–7), revealing its significant role in global gene expression. Among the NNS complex, Nrd1 is an RNA-binding protein involved in RNA polymerase II transcription termination. In this study, we characterized the orthologous NrdA in Aspergillus species, including A. kawachii, A. oryzae, A. nidulans, and A. fumigatus, particularly in the early developmental stage. Examination of protein domains and phylogenetic analysis of NrdA indicates that Nrd1 orthologs are well conserved in the genus Aspergillus and are closer to Shiz. pombe Seb1 than to S. cerevisiae Nrd1 (see Fig. S1 and S2 in the supplemental material). We demonstrated that nrdA is an essential gene in A. kawachii using the nrdA conditional expression strain. This result is consistent with previous reports stating that NRD1 and seb1 are essential genes in S. cerevisiae and Schiz. pombe, respectively (28, 29). This indicates that the NrdA-related gene regulation plays an essential role not only in the growth of yeast but also in that of filamentous fungi.
In this study, we employed the RIP-seq to identify the NrdA-interacting transcripts in A. kawachii. We predicted NrdA–mRNA interactions by identifying the mRNA significantly enriched by RIP based on the following criteria: log2 fold change > 0 and q-value < 0.05 (see Data Set S1 in the supplemental material). This analysis identified 3,676 mRNA transcripts that may interact with NrdA. Applying the criteria of log2 fold change > 0 and q-value < 0.05 for enrichment, we observed a decrease in the number of predicted mRNA-NrdA interactions as the log2 fold change approached 0, resulting in a minimum log2 fold change of 0.18 (Fig. 4B, see Fig. S5 in the supplemental material). Although the interaction of NrdA with these transcripts requires further verification, the 3,676 represent 32% of the total 11,474 predicted coding sequences of A. kawachii (using conventional cutoffs of log2 fold change > 1 and q-value < 0.05, we find 2,873 mRNA transcripts that may interact with NrdA). This is consistent with the estimation that Nrd1 and Nab3 directly regulate a variety of protein-coding genes, representing 20%–30% of the protein-coding transcripts of S. cerevisiae (5). In addition, 3,755 mRNA molecules, which are 33% of the total coding sequences of A. kawachii, were identified with a log2 fold change <0 and q-value <0.05. These similar percentages of transcripts may undergo competitive reduction due to the lack of interaction with NrdA.
GO analysis indicated that the overexpression of nrdA causes changes in the mRNA levels of genes related to secondary metabolism in A. kawachii. Thus, we further studied the effect of nrdA overexpression in other Aspergillus species. It is important to note that A. kawachii does not produce known toxigenic secondary metabolites such as ochratoxin A and fumonisin B, making it safe to be used in the food and beverage industry (30–32). The non-mycotoxin productivity is due to the absence of genes related to ochratoxin and fumonisin biogenesis in the genome of A. kawachii (31, 32). Because A. kawachii is used for producing shochu, sake, and fermented food, non-mycotoxin productivity is crucial. In addition, A. kawachii is an albino mutant of the black koji fungus A. luchuensis due to a mutation in the PKS gene (pksP) involved in the production of 1,8-dihydroxynaphthalene-melanin (33, 34).
Overexpression of nrdA reduced the production of sterigmatocystin and penicillin in A. nidulans (Fig. 5C and D), as well as helvolic acid and pyripyropene A in A. fumigatus (Fig. 6C), and kojic acid and penicillin in A. oryzae (Fig. 7A and D) at earlier cultivation times. These results were possibly accompanied by consistent changes in the mRNA levels of relevant genes (Fig. 5E, 6D, 7C, and E). It should be noted that these observations were made during the early growth stages of 24 and 48 hours of cultivation. For example, the A. nidulans OE-nrdA strain produced lower amounts of penicillin at 24 and 48 hours, but not at 72 hours compared to the control strain (Fig. 5C). In addition, sterigmatocystin production recovered over time (Fig. 5D). This might be because the overexpression of nrdA was successfully achieved only at 12 h, an earlier growth stage; however, the nrdA gene expression level decreased to that of the control strain at the later growth stages of 24 and 48 h (Fig. 5E). Furthermore, we confirmed that there was no significant difference in nrdA expression between the control and OE-nrdA strains during the later cultivation stages at 74 and 96 h (data not shown). The A. nidulans OE-nrdA strain was constructed using the promoter of the glyceraldehyde-3-phosphate dehydrogenase-encoding gpdA gene. Therefore, we also constructed another A. nidulans strain overexpressing nrdA using the Tet-On promoter; however, overexpression of nrdA failed at the later growth stages (data not shown). Thus, the effect of NrdA overexpression on the production of secondary metabolites remains unclear at the later growth stages. In S. cerevisiae, Nrd1 autoregulates its expression level (35, 36). An efficient autoregulation mechanism exists where the synthesis rate of the full-length NRD1 transcript is inversely correlated to the amount of Nrd1 protein. Such a regulatory mechanism may also explain why nrdA overexpression does not persist in A. nidulans.
How does NrdA affect the production of secondary metabolites? One hypothesis is that NrdA may be directly involved in silencing secondary metabolite gene clusters via an NNS-related transcription termination system. This is supported by the observation that overexpression of nrdA suppressed the gene expression levels of ipnA, aflR, and stcU in A. nidulans (Fig. 5E), helA and pyr2 in A. fumigatus (Fig. 6D), and ipnA in A. oryzae (Fig. 7E). Another hypothesis is that these genes may be indirectly regulated via transcription factors and/or histone modification and chromatin remodeling, which often regulate the production of secondary metabolites in fungi (37). For example, the transcription factor BrlA regulates the production of fumagillin, helvolic acid, and pyripyropene A in A. fumigatus (38). The production of sterigmatocystin and penicillin is regulated by the putative methyltransferase LaeA in A. nidulans (39). The production of penicillin and kojic acid is regulated by histone deacetylase Hst4 and LaeA (40, 41).
In S. cerevisiae, Nrd1 and exosome-dependent RNA degradation leads to gene silencing by histone modification. The euchromatin marks, such as histone H3 trimethylated at lysine 4 (H3K4me3) and histone H3 acetylated at lysine 9 (H3K9ac), at the non-transcribed spacer locus of ribosomal DNA, a heterochromatin region, are enriched by the depletion of Nrd1 (42). Recently, it was also reported that heterochromatin assembly occurs by the pausing of RNA polymerase II promoted by Seb1 in Schiz. pombe (43). Thus, overexpression of NrdA might contribute to the formation of heterochromatin at the secondary metabolite gene cluster regions in Aspergillus species.
We demonstrated that overexpression of nrdA causes changes in global gene expression and concomitant changes in secondary metabolite production in Aspergillus species at the early growth stage. However, it remains unclear whether such NrdA-dependent regulation is its intrinsic physiological function. Our previous transcriptomic profiles of A. kawachii showed no changes in nrdA expression during solid-state culture on steamed barley (10). In addition, nrdA expression was not altered by disruptions of laeA and hepA, genes encoding global regulators involved in various phenomena, including secondary metabolism (44, 45). On the other hand, we found that the gene expression level of nrdA decreased with the disruption of sirD, which encodes the NAD+-dependent class III histone deacetylase (46). SirD (Sirtuin E in A. nidulans and A. fumigatus) is involved in the transition from primary to secondary metabolism (46–48), implying a functional relationship between NrdA and SirD. Thus, it is necessary to analyze the physiological significance of NrdA in secondary metabolite production, especially in the later stages of culture, focusing on SirD-dependent nrdA regulation.
This study was initiated due to an interest in the nrdA genes localized in the syntenic region with citA and yhmA on the genome. However, the transcripts of citA (AKAW_06279) and yhmA (AKAW_06280) were not predicted to interact with NrdA (see Data Set S1 in the supplemental material). In addition, citA and yhmA are not essential genes in A. kawachii (11, data not shown), indicating that they are not responsible for the lethality caused by nrdA disruption. We could not determine the reason for the co-localization of nrdA with citA and yhmA in the subdivision Pezizomycotina; however, NrdA plays a significant role in global gene regulation and is involved in the production of secondary metabolites in Aspergillus species. CAP is regulated by the secondary metabolism regulator LaeA in Aspergillus niger, Aspergillus carbonarius, and A. kawachii (44, 49, 50). Therefore, NrdA may be linked to CAP via the regulation of secondary metabolism.
Although the NNS-associated RNA surveillance system has been extensively investigated in the yeasts S. cerevisiae and Schiz. pombe, the present findings may enhance the understanding of NNS-associated production of secondary metabolites in Aspergillus species, including industrially and clinically important fungi. Needless to say, the functional characterization of orthologs of other NNS complex components such as Nab3 and Sen1 is required for a better understanding of the importance of the NNS-related transcriptional termination pathway in the genus Aspergillus.
MATERIALS AND METHODS
Strains and culture conditions
In this study, A. kawachii SO2 (51), A. nidulans A26 (Fungal Genetics Stock Center [FGSC; Manhattan, KS]), A. fumigatus A1151 (FGSC), and A. oryzae ΔligD plus pGNA (52, 53) were used as parental strains (Table 2). Control strains were defined to show an identical auxotrophic background for comparison with the respective nrdA overexpression strains.
TABLE 2.
Aspergillus strains used in this studyb
| Strain name or description | Genotype | Reference |
|---|---|---|
| Aspergillus luchuensis mut. kawachii | ||
| IFO 4308 | Wild type | IFO |
| SO2 | ligD− argB::hph sC− | (51) |
| CK3a | ligD− argB::hph sC− pVG2.2ANsC | This study |
| Tet-nrdA | ligD− argB::hph sC− pVG2.2ANsC nrdA::argB-Tet-nrdA | This study |
| Tet-nrdA plus GFP-nrdA plus H2B-mRFP | ligD− argB::hph sC− pVG2.2ANsC nrdA::argB-Tet-nrdA pPTRI-GFP-nrdA pGbar-H2B-mRFP | This study |
| Tet-S-nrdA | ligD- argB::hph sC− pVG2.2ANsC nrdA::argB-Tet-S-nrdA | This study |
| Aspergillus nidulans | ||
| A26a | veA1 biA1 | FGSC |
| OE-nrdA | veA1 biA1 pPTRI-PgpdA-nrdA | This study |
| Aspergillus fumigatus | ||
| A1151a | akuB::pyrG | FGSC |
| OE-nrdA | akuB::pyrG nrdA::ptrA-PgpdA-nrdA | This study |
| Aspergillus oryzae | ||
| ΔligD plus pGNA | sC− niaD− ligD::sC pGNA | (53) |
| OE-nrdA | sC− niaD− ligD::sC pGNA nrdA::ptrA-PgpdA-nrdA | This study |
These strains were used as controls.
IFO, Institute for Fermentation, Osaka Japan; FGSC, Fungal Genetics Stock Center.
Strains were cultivated in minimal medium (54; FGSC [http://www.fgsc.net/methods/anidmed.html]) with or without 0.211% (wt/vol) arginine and/or 0.15% (wt/vol) methionine, and/or 0.1 µg/mL pyrimethamine. CAP medium (11), kojic acid production medium with 1 mM ferric ion (27), or YES (15% [wt/vol] sucrose, 2% [wt/vol] yeast extract) medium were used appropriately for the fungal growth experiments. Minimal and CAP media were adjusted to the required pH with NaOH and HCl, respectively. For the cultivation of A. nidulans strains, a minimal medium was supplemented with 5 µg/mL biotin.
Construction of A. kawachii Tet-nrdA and Tet-S-nrdA strains
To achieve Dox-inducible conditional expression of the nrdA gene, 2 kb of the 5′-end of nrdA, 2.2 kb of argB, 0.8 kb of cgrA terminator and Tet-On promoter, and 1.8 kb of a part of the ORF region of nrdA were constructed by recombinant PCR using the primer pairs AKtet-nrdA-FC/AKtet-nrdA-R1, AKtet-nrdA-F2/AKtet-nrdA-R2, AKtet-nrdA-F3/AKtet-nrdA-R3, and AKtet-nrdA-F4/AKtet-nrdA-RC, respectively (see Table S1 in the supplemental material). For amplification of the argB gene and Tet-On promoter, A. nidulans A26 genomic DNA and plasmid pVG2.2ANsC were used as template DNA, respectively (11, 55). The resultant DNA fragment was amplified with the primers AKtet-nrdA-F1 and AKtet-nrdA-R4 and used to transform the A. kawachii strain CK3, which carried pVG2.2ANsC, yielding the Tet-nrdA strain. Transformants were selected on a minimal agar medium without arginine. The introduction of this cassette into the target locus was confirmed with PCR using the primer pairs AKtet-nrdA-FC and AKtet-nrdA-RC (see Fig. S6 in the supplemental material).
To confirm the functional expression of S-tagged NrdA, we constructed an S-NrdA expression strain (data not shown). The 2 kb of the 5′-end of nrdA, 1.8 kb of ptrA, and 1.8 kb of a part of the ORF region of S-nrdA were constructed by recombinant PCR using the primer pairs Aktet-nrdA-FC/AkS-nrdA-ptrA-R1, AkS-nrdA-ptrA-F2/AkS-nrdA-ptrA-R2, and AkS-nrdA-ptrA-F3/AKtet-nrdA-RC, respectively (see Table S1 in the supplemental material). For amplification of the ptrA gene, pPTR I (Takara bio, Shiga, Japan) (56) was used as template DNA. The resultant DNA fragment was amplified with the primers AKtet-nrdA-F1 and AKtet-nrdA-R4 and used to transform the A. kawachii strain SO2, yielding the S-nrdA strain. Transformants were selected on a minimal agar medium with pyrithiamine.
To achieve Dox-inducible conditional expression of the S-nrdA gene, 2 kb of the 5′-end of nrdA, 2.2 kb of argB, 0.8 kb of cgrA terminator and Tet-On promoter, and 2 kb of a part of ORF region of nrdA were constructed by recombinant PCR using the primer pairs AKtet-nrdA-FC/AKtet-S-nrdA-R3, and AKtet-S-nrdA-F4/AKtet-nrdA-RC, respectively (see Table S1 in the supplemental material). For amplification of the 5′-end nrdA-argB-Tet-On promoter and S-nrdA, the genomic DNA of strain Tet-nrdA and strain S-nrdA were used as template DNA, respectively. The resultant DNA fragment was amplified with the primers AKtet-nrdA-F1 and AKtet-nrdA-R4 and used to transform the A. kawachii strain CK3, yielding the Tet-S-nrdA strain. Transformants were selected on a minimal agar medium without arginine. The introduction of this cassette into the target locus was confirmed with PCR using the primer pairs AKtet-nrdA-FC and AKtet-nrdA-RC (see Fig. S6 in the supplemental material). The expression of S-tagged NrdA was confirmed by purification using S-protein agarose (Merck Millipore, Darmstadt, Germany) as previously described (11, 57). The purified protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting analysis using an anti-S-tag antibody (Medical and Biological Laboratories, Nagoya, Japan) (Fig. 3B).
Construction of an A. kawachii strain expressing GFP-NrdA and H2B-mRFP
The plasmid pPTR I (Takara Bio), which carries the ptrA gene, was used to construct the expression vector for GFP-NrdA. The 5′-end of nrdA (until start codon) and gfp and nrdA ORF (without start codon) were amplified by PCR using the primer pairs pPTRI-gfp-nrdA-F1/pPTRI-gfp-nrdA-R1, pPTRI-gfp-nrdA-F2/pPTRI-gfp-nrdA-R2, and pPTRI-gfp-nrdA-F3/pPTRI-gfp-nrdA-R3, respectively. For the amplification of gfp, plasmid pFNO3 (58) was used as template DNA. The resultant DNA fragment was amplified with the primers pPTRI-gfp-nrdA-inf-F/pPTRI-gfp-nrdA-inf-R (see Table S1 in the supplemental material). The amplified fragment was cloned into the SmaI site of pPTR I using an In-Fusion HD cloning kit (Takara Bio). The pPTR I-GFP-nrdA plasmid was used to transform the A. kawachii strain Tet-nrdA, yielding the GFP-NrdA strain. Transformants were selected on minimal agar medium with pyrithiamine. After fluorescence microscopy testing, the pGbar-H2B-mRFP plasmid (46) was used to transform the A. kawachii strain GFP-NrdA, yielding the GFP-NrdA H2B-mRFP strain. Transformants were selected on a minimal agar medium with glufosinate extracted from the herbicide Basta (Bayer Crop Science, Bayer Japan, Tokyo, Japan).
Fluorescence microscopy
A strain expressing GFP-NrdA and H2B-mRFP was cultured in minimal medium supplemented with arginine. After cultivation in minimal medium supplemented with arginine for 12 h, the mycelia were observed under a DMI6000B inverted-type fluorescent microscope (Leica Microsystems, Wetzlar, Germany). Image contrast was adjusted using the LAS AF Lite software, version 2.3.0, build 5131 (Leica Microsystems).
Measurement of citric acid
To measure the levels of extracellular citric acid, conidia (2 × 107 cells) of the A. kawachii control strain were inoculated into 100 mL of YES medium and precultivated with shaking (180 rpm) at 30°C for 16 h. Subsequently, they were transferred to 50 mL of CAP medium with arginine and further cultivated with shaking (163 rpm) at 30°C for 48 h. Prior to their transfer to CAP medium, the mycelia were washed using a fresh CAP medium. The Tet-nrdA strain was precultured in YES medium with 1 µg/mL Dox and transferred to CAP medium with or without Dox. The culture supernatant was filtered through a PTFE filter (pore size: 0.2 µm) (Toyo Roshi Kaisha, Tokyo, Japan) and used as the extracellular fraction.
The concentration of citric acid was determined using a Prominence HPLC system (Shimadzu, Kyoto, Japan) equipped with a CDD-10AVP conductivity detector (Shimadzu). The organic acids were separated with tandem Shimadzu Shim-pack SCR-102H columns (300 × 8 mm I.D.; Shimadzu) at 50°C using 4 mM p-toluenesulfonic acid monohydrate as the mobile phase at a flow rate of 0.8 mL/min. The flow rate of the post-column reaction solution (4 mM p-toluenesulfonic acid monohydrate, 16 mM bis-Tris, and 80 µM ethylenediaminetetraacetic acid [EDTA]) was 0.8 mL/min.
RNA preparation
To identify the transcriptome of A. kawachii control and Tet-S-nrdA strains, conidia (2 × 107 cells) were inoculated into 100 mL of YES medium with 1 µg/mL Dox (for Tet-S-nrdA strain) and precultured for 14 h at 30°C. Subsequently, they were transferred to 50 mL of YES medium with or without Dox (for Tet-S-nrdA strain) and further cultivated with shaking (163 rpm) at 30°C for 24 h. Prior to their transfer to YES medium with or without Dox, the mycelia were washed with fresh YES medium. Following incubation, the mycelia were collected and ground to a powder in the presence of liquid nitrogen. Total RNA was extracted using RNAiso Plus (Takara Bio) according to the instructions provided by the manufacturer and quantified using a NanoDrop-8000 (Thermo Fisher Scientific).
RIP assay
To identify NrdA interacting RNA, A. kawachii Tet-S-nrdA strain was cultivated using YES medium with Dox under the condition described for the RNA preparation. The mycelia were collected and ground to a powder in the presence of liquid nitrogen. The powdered mycelia (1 g wet weight) were dissolved in 13 mL of ice-cold nuclear extraction buffer (25 mM N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid [HEPES; pH 6.8], 1 M sorbitol, 250 µg/mL phenylmethylsulfonyl fluoride [PMSF], cOmplete [EDTA-free protease inhibitor cocktail; Roche, Basel, Switzerland]) and vigorously mixed using a vortexer. Cell debris was removed by filtration using Miracloth, and the solution was centrifuged at 10,000 × g at 4°C for 15 min. The supernatant was removed and the pellet was dissolved in 500 µL of ice-cold nuclear solubilization buffer (25 mM HEPES [pH 6.8], 1 M sorbitol, 0.5% NP-40, 250 µg/mL PMSF, cOmplete [EDTA-free protease inhibitor cocktail; Roche]). Following incubation for 30 min at 4°C, the debris was removed by centrifugation at 2,000 × g at 4°C for 15 min. Twenty-five microliters of protein A Sepharose beads (50% slurry contained phosphate-buffered saline buffer) (GE Healthcare, Chicago, IL) was added to the resultant supernatant, and the resulting mixture was gently mixed for 1 h at 4°C using a rotator. The mixture was separated by centrifugation at 2,000 × g at 4°C for 1 min, and 25 µL of anti-S-tag antibody (Medical and Biological Laboratories) or normal rabbit IgG (Medical and Biological Laboratories) cross-linked protein A Sepharose was added to the supernatant. Subsequently, these mixtures were gently mixed for 3 h at 4°C using a rotator. The RNA interacted with NrdA and was purified using RiboCluster Profiler (Medical and Biological Laboratories) according to the instructions provided by the manufacturer and quantified using a NanoDrop-8000 (Thermo Fisher Scientific).
Library preparation, sequencing, and data analysis for RNA-seq and RIP-seq were performed by Kabushiki Kaisha DNAFORM (Yokohama, Japan). All RNA samples were treated with RiboZero (Human/Mouse/Rat) (Illumina, San Diego, CA) for the depletion of ribosomal RNA. All RNA-seq and RIP-seq experiments were performed thrice with RNA samples obtained from independently prepared mycelia using the NextSeq 500 system (Illumina) and mapped to the A. kawachii IFO 4308 genome (32) using a pipeline of trimming (trim_galore [http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/], trimmomatic (59), cutadapt (60)), mapping (STAR) (61), counting gene levels (featureCount) (62), differential expression analysis (DEseq2) (63), and clustering analysis (MBCluster.Seq) (64). A gene set of A. kawachii was converted to a homologous gene set of A. niger to perform the GO term enrichment analysis using the GO Term Finder of the Aspergillus genome database (AspGD) (65). This species was used because A. niger is closely related to A. kawachii (9, 66, 67).
Construction of the A. nidulans nrdA overexpression strain
The plasmid pPTR I (Takara Bio) was used to construct the overexpression vector for the A. nidulans nrdA gene (locus tag, AN8276). The A. nidulans gpdA promoter and nrdA were amplified by PCR using the primer pairs pPTRI-PgpdA-nrdA-inf-F1/pPTRI-PgpdA-nrdA-inf-R1 and pPTRI-PgpdA-nrdA-inf-F2/pPTRI-PgpdA-nrdA-inf-R2 (see Table S1 in the supplemental material). The amplified fragments were cloned into the SmaI site of pPTR I using an In-Fusion HD cloning kit (Takara Bio). Transformants were selected on a minimal agar medium with pyrithiamine.
Construction of the A. fumigatus nrdA overexpression strain
For the overexpression analysis of nrdA in A. fumigatus (locus tag, AFUB_052760), a gene replacement cassette encompassing a homology arm at the 5′ end of nrdA, ptrA selection marker, A. nidulans gpdA promoter, and homology arm at the nrdA locus was constructed through recombinant PCR using the primer pairs AfPgpdA-nrdA-FC/AfPgpdA-nrdA-R1, AfPgpdA-nrdA-F2/PgpdA-nrdA-R2, AfPgpdA-nrdA-F3/PgpdA-nrdA-R3, and AfPgpdA-nrdA-F4/AfPgpdA-nrdA-RC, respectively (see Table S1 in the supplemental material). For the amplification of DNA fragments, A. fumigatus FGSC A1151 genomic DNA, A. nidulans A26 genomic DNA, or plasmid pPTR I (Takara Bio) were used as template DNA. The resultant DNA fragments amplified with primers AfPgpdA-nrdA-F1/AfPgpdA-nrdA-R4 were used to transform the A. fumigatus FGSC A1151, yielding the A. fumigatus OE-nrdA strain. Transformants were selected on a minimal agar medium supplemented with 0.1 µg/mL pyrithiamine. Introduction of the ptrA and A. nidulans gpdA promoter into the target locus was confirmed by PCR using primers AfPgpdA-nrdA-FC/AfPgpdA-nrdA-RC (see Fig. S7 in the supplemental material).
Construction of the A. oryzae nrdA overexpression strain
For the overexpression analysis of nrdA in A. oryzae (locus tag, AO090102000629), a gene replacement cassette encompassing a homology arm at the 5′ end of nrdA, ptrA selection marker, A. nidulans gpdA promoter, and homology arm at the nrdA locus of A. oryzae was constructed with recombinant PCR using the primer pairs AoPgpdA-nrdA-FC/AoPgpdA-nrdA-R1, AoPgpdA-nrdA-F2/PgpdA-nrdA-R2, AoPgpdA-nrdA-F3/PgpdA-nrdA-R3, and AoPgpdA-nrdA-F4/AoPgpdA-nrdA-RC, respectively (see Table S1 in the supplemental material). For the amplification of DNA fragments, A. oryzae RIB40 wild-type genomic DNA, A. nidulans A26 genomic DNA, and plasmid pPTR I (Takara Bio) were used as template DNA. These resultant DNA fragments amplified with primers AoPgpdA-nrdA-F1/AoPgpdA-nrdA-R4 were used to transform the A. oryzae strain ΔligD plus pGNA (10, 43), yielding the A. oryzae strain OE-nrdA. Transformants were selected on a minimal agar medium supplemented with 0.1 µg/mL pyrithiamine. Introduction of the ptrA and A. nidulans gpdA promoter into the target locus was confirmed by PCR using primers AoPgpdA-nrdA-FC/AoPgpdA-nrdA-RC (see Fig. S8 in the supplemental material).
Analysis of secondary metabolites by liquid chromatography-mass spectrometry
Conidia (2 × 107 cells) were inoculated into 100 mL of YES medium and precultivated with shaking (180 rpm) at 30°C for 16 h. Subsequently, they were transferred to 50 mL of minimal medium supplemented with biotin (for A. nidulans strains) or with 5% (vol/vol) FBS (for A. fumigatus strains) and further cultivated with shaking (163 rpm) at 30°C for 24 or 48 h. The mycelia were collected and used to measure the weight of freeze-dried mycelia. Culture supernatant (5 mL) was collected and mixed with an equal volume of ethyl acetate for A. nidulans or chloroform for A. fumigatus. The mixture was centrifuged at 10,000 × g at 4°C for 15 min. The ethyl acetate fraction was collected, evaporated by N2 gas spraying, and resuspended in 100 µL of acetonitrile. The concentration of sterigmatocystin, fumagillin, helvolic acid, and pyripyropene A was determined using a Prominence ultra-high-performance liquid chromatograph system (Shimadzu) equipped with a 3200 QTRAP system (AB SCIEX, Framingham, MA) in the multiple reaction monitoring modes. The secondary metabolites were separated with an ACQUITY UPLC CSH C18 Column (1.7 µm, 2.1 mm × 50 mm) (Waters, Milford, MA) at 40°C using 0.05% formic acid in acetonitrile as the organic phase and 0.05% formic acid in water as the aqueous phase at a flow rate of 0.2 mL/min. The solvent gradient started at 20% organic for 2 min, followed by a linear increase to 60% organic over 10 min, a linear increase to 100% organic over 1 min, and final maintenance at 100% organic for 20 min.
Penicillin bioassay
Conidia (2 × 107 cells) of the A. nidulans control and OE-nrdA strains and A. oryzae control and OE-nrdA strains were inoculated into 100 mL of YES medium and precultivated with shaking (180 rpm) at 30°C for 16 h. Subsequently, they were transferred to 50 mL of minimal medium supplemented with biotin and further cultivated with shaking (163 rpm) at 30°C for 24, 48, or 72 h. Culture supernatant (5 mL) was evaporated by freeze drying and resuspended in 500 µL of water. Penicillin bioassay was performed using Kocuria rhizophila (NBRC 12708) as previously reported (39).
Kojic acid assay
Conidia (1 × 104 cells) of A. oryzae strains were inoculated onto kojic acid production agar medium with ferric ion (chelation by kojic acid changes the color of the medium to red) (27). After cultivation at 30°C for 5 days, the color of the medium was observed to evaluate the production of kojic acid.
mRNA level analysis
To evaluate the expression level of S-nrdA in the A. kawachii Tet-S-nrdA strain, the Tet-S-nrdA strain was precultivated in YES medium with Dox for 16 h. Next, the mycelia were transferred to YES medium with or without Dox and further cultivated for 4, 6, 12, or 24 h with shaking (163 rpm) at 30°C. In addition, the A. kawachii control strain was precultivated in the YES medium for 16 h. The mycelia were transferred to the YES medium and further cultivated for 24 h.
To evaluate the gene expression level involved in the production of secondary metabolites, A. nidulans, A. fumigatus, and A. oryzae strains were cultivated under the conditions described for the analysis of secondary metabolites. For the analysis of transcripts related to the production of a kojic acid in A. oryzae, conidia (2 × 107 cells) were inoculated onto kojic acid production medium with ferric ion (27) and incubated with shaking (163 rpm) at 30°C for 5 days. The mycelia were collected from the liquid culture using gauze and ground to a powder in the presence of liquid nitrogen.
Total RNA was extracted using RNAiso Plus (Takara Bio) according to the instructions provided by the manufacturer and quantified using a NanoDrop-8000 (Thermo Fisher Scientific). cDNA was synthesized from total RNA using a PrimeScript Perfect real-time reagent kit (Takara Bio) according to the instructions provided by the manufacturer. Real-time reverse transcription-PCR was performed using a Thermal Cycler Dice real-time system MRQ (Takara Bio) with TB Green Premix Ex Taq II (Tli RNaseH Plus) (Takara Bio). The following primer sets were used: ANnrdA-RT-F and ANnrdA-RT-R for A. nidulans nrdA, ANipnA-RT-F and ANipnA-RT-R for A. nidulans ipnA, ANaflR-RT-F and ANaflR-RT-R for A. nidulans aflR, ANstcU-RT-F and ANstcU-RT-R for A. nidulans stcU, ANacnA-RT-F and ANacnA-RT-R for A. nidulans acnA, AFnrdA-RT-F and AFnrdA-RT-R for A. fumigatus nrdA, AFfumR-RT-F and AFfumR-RT-R for A. fumigatus fumR, AFhelA-RT-F and AFhelA-RT-R for A. fumigatus helA, AFpyr2-RT-F and AFpyr2-RT-R for A. fumigatus pyr2, AFact1-RT-F and AFact1-RT-R for A. fumigatus act1, AOnrdA-RT-F and AOnrdA-RT-R for A. oryzae nrdA, AOkojR-RT-F and AOkojR-RT-R for A. oryzae kojR, AOkojA-RT-F and AOkojA-RT-R for A. oryzae kojA, AOkojT-RT-F and AOkojT-RT-R for A. oryzae kojT, AOipnA-RT-F and AOipnA-RT-R for A. oryzae ipnA, and AOactA-RT-F and AOactA-RT-R for A. oryzae actA (see Table S1 in the supplemental material). The gene expression levels of acnA, act1, and actA were used to calibrate those of A. nidulans, A. fumigatus, and A. oryzae, respectively.
ACKNOWLEDGMENTS
We thank the Division of Instrumental Analysis Research Support Center of Kagoshima University for technical support. This study was supported in part by KAKENHI (grant numbers: 16K07672, 18K05394, 19K05773, 22H02246, and 23K23513), the Institute for Fermentation, Osaka (IFO), and a research grant from Kagoshima University based on a scholarship donation from SUNUS Co., Ltd. C.K. and K.H. received a Grant-in-Aid for JSPS Research Fellows (grant number: 17J02753 and 24KJ1839, respectively).
Contributor Information
Taiki Futagami, Email: k2130730@kadai.jp.
Aaron P. Mitchell, University of Georgia, Athens, Georgia, USA
DATA AVAILABILITY
The data obtained from the RNA-seq and RIP-seq analyses in this study were deposited in the Gene Expression Omnibus under accession number GSE164392.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msphere.00849-24.
Differential analysis of RNA-seq and RIP-seq.
Fig. S1 to S8; Tables S1 and S2.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Kuehner JN, Pearson EL, Moore C. 2011. Unravelling the means to an end: RNA polymerase II transcription termination. Nat Rev Mol Cell Biol 12:283–294. doi: 10.1038/nrm3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arndt KM, Reines D. 2015. Termination of transcription of short noncoding RNAs by RNA polymerase II. Annu Rev Biochem 84:381–404. doi: 10.1146/annurev-biochem-060614-034457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Darby MM, Serebreni L, Pan X, Boeke JD, Corden JL. 2012. The Saccharomyces cerevisiae Nrd1-Nab3 transcription termination pathway acts in opposition to Ras signaling and mediates response to nutrient depletion. Mol Cell Biol 32:1762–1775. doi: 10.1128/MCB.00050-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merran J, Corden JL. 2017. Yeast RNA-binding protein Nab3 regulates genes involved in nitrogen metabolism. Mol Cell Biol 37:e00154-17. doi: 10.1128/MCB.00154-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Webb S, Hector RD, Kudla G, Granneman S. 2014. PAR-CLIP data indicate that Nrd1-Nab3-dependent transcription termination regulates expression of hundreds of protein coding genes in yeast. Genome Biol 15:R8. doi: 10.1186/gb-2014-15-1-r8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lemay J-F, Marguerat S, Larochelle M, Liu X, van Nues R, Hunyadkürti J, Hoque M, Tian B, Granneman S, Bähler J, Bachand F. 2016. The Nrd1-like protein Seb1 coordinates cotranscriptional 3’ end processing and polyadenylation site selection. Genes Dev 30:1558–1572. doi: 10.1101/gad.280222.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wittmann S, Renner M, Watts BR, Adams O, Huseyin M, Baejen C, El Omari K, Kilchert C, Heo DH, Kecman T, Cramer P, Grimes JM, Vasiljeva L. 2017. The conserved protein Seb1 drives transcription termination by binding RNA polymerase II and nascent RNA. Nat Commun 8:14861. doi: 10.1038/ncomms14861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suganuma T, Fujita K, Kitahara K. 2007. Some distinguishable properties between acid-stable and neutral types of alpha-amylases from acid-producing koji. J Biosci Bioeng 104:353–362. doi: 10.1263/jbb.104.353 [DOI] [PubMed] [Google Scholar]
- 9. Yamada O, Takara R, Hamada R, Hayashi R, Tsukahara M, Mikami S. 2011. Molecular biological researches of Kuro-Koji molds, their classification and safety. J Biosci Bioeng 112:233–237. doi: 10.1016/j.jbiosc.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 10. Futagami T, Mori K, Wada S, Ida H, Kajiwara Y, Takashita H, Tashiro K, Yamada O, Omori T, Kuhara S, Goto M. 2015. Transcriptomic analysis of temperature responses of Aspergillus kawachii during barley koji production. Appl Environ Microbiol 81:1353–1363. doi: 10.1128/AEM.03483-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kadooka C, Izumitsu K, Onoue M, Okutsu K, Yoshizaki Y, Takamine K, Goto M, Tamaki H, Futagami T. 2019. Mitochondrial citrate transporters CtpA and YhmA are required for extracellular citric acid accumulation and contribute to cytosolic acetyl coenzyme A generation in Aspergillus luchuensis mut. kawachii. Appl Environ Microbiol 85:e03136-18. doi: 10.1128/AEM.03136-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nützmann HW, Scazzocchio C, Osbourn A. 2018. Metabolic gene clusters in eukaryotes. Annu Rev Genet 52:159–183. doi: 10.1146/annurev-genet-120417-031237 [DOI] [PubMed] [Google Scholar]
- 13. Rokas A, Wisecaver JH, Lind AL. 2018. The birth, evolution and death of metabolic gene clusters in fungi. Nat Rev Microbiol 16:731–744. doi: 10.1038/s41579-018-0075-3 [DOI] [PubMed] [Google Scholar]
- 14. Steinmetz EJ, Brow DA. 1998. Control of pre-mRNA accumulation by the essential yeast protein Nrd1 requires high-affinity transcript binding and a domain implicated in RNA polymerase II association. Proc Natl Acad Sci U S A 95:6699–6704. doi: 10.1073/pnas.95.12.6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kubicek K, Cerna H, Holub P, Pasulka J, Hrossova D, Loehr F, Hofr C, Vanacova S, Stefl R. 2012. Serine phosphorylation and proline isomerization in RNAP II CTD control recruitment of Nrd1. Genes Dev 26:1891–1896. doi: 10.1101/gad.192781.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vasiljeva L, Kim M, Mutschler H, Buratowski S, Meinhart A. 2008. The Nrd1-Nab3-Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nat Struct Mol Biol 15:795–804. doi: 10.1038/nsmb.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franco-Echevarría E, González-Polo N, Zorrilla S, Martínez-Lumbreras S, Santiveri CM, Campos-Olivas R, Sánchez M, Calvo O, González B, Pérez-Cañadillas JM. 2017. The structure of transcription termination factor Nrd1 reveals an original mode for GUAA recognition. Nucleic Acids Res 45:10293–10305. doi: 10.1093/nar/gkx685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conrad NK, Wilson SM, Steinmetz EJ, Patturajan M, Brow DA, Swanson MS, Corden JL. 2000. A yeast heterogeneous nuclear ribonucleoprotein complex associated with RNA polymerase II. Genetics 154:557–571. doi: 10.1093/genetics/154.2.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Rourke TW, Loya TJ, Head PE, Horton JR, Reines D. 2015. Amyloid-like assembly of the low complexity domain of yeast Nab3. Prion 9:34–47. doi: 10.1080/19336896.2014.997618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramón D, Carramolino L, Patiño C, Sánchez F, Peñalva MA. 1987. Cloning and characterization of the isopenicillin N synthetase gene mediating the formation of the β-lactam ring in Aspergillus nidulans. Gene 57:171–181. doi: 10.1016/0378-1119(87)90120-x [DOI] [PubMed] [Google Scholar]
- 21. Yu JH, Butchko RA, Fernandes M, Keller NP, Leonard TJ, Adams TH. 1996. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr Genet 29:549–555. doi: 10.1007/BF02426959 [DOI] [PubMed] [Google Scholar]
- 22. Keller NP, Kantz NJ, Adams TH. 1994. Aspergillus nidulans verA is required for production of the mycotoxin sterigmatocystin. Appl Environ Microbiol 60:1444–1450. doi: 10.1128/aem.60.5.1444-1450.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown DW, Yu JH, Kelkar HS, Fernandes M, Nesbitt TC, Keller NP, Adams TH, Leonard TJ. 1996. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc Natl Acad Sci U S A 93:1418–1422. doi: 10.1073/pnas.93.4.1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dhingra S, Lind AL, Lin HC, Tang Y, Rokas A, Calvo AM. 2013. The fumagillin gene cluster, an example of hundreds of genes under veA control in Aspergillus fumigatus. PLoS ONE 8:e77147. doi: 10.1371/journal.pone.0077147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lv JM, Hu D, Gao H, Kushiro T, Awakawa T, Chen GD, Wang CX, Abe I, Yao XS. 2017. Biosynthesis of helvolic acid and identification of an unusual C-4-demethylation process distinct from sterol biosynthesis. Nat Commun 8:1644. doi: 10.1038/s41467-017-01813-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Itoh T, Tokunaga K, Matsuda Y, Fujii I, Abe I, Ebizuka Y, Kushiro T. 2010. Reconstitution of a fungal meroterpenoid biosynthesis reveals the involvement of a novel family of terpene cyclases. Nat Chem 2:858–864. doi: 10.1038/nchem.764 [DOI] [PubMed] [Google Scholar]
- 27. Terabayashi Y, Sano M, Yamane N, Marui J, Tamano K, Sagara J, Dohmoto M, Oda K, Ohshima E, Tachibana K, Higa Y, Ohashi S, Koike H, Machida M. 2010. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal Genet Biol 47:953–961. doi: 10.1016/j.fgb.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 28. Steinmetz EJ, Brow DA. 1996. Repression of gene expression by an exogenous sequence element acting in concert with a heterogeneous nuclear ribonucleoprotein-like protein, Nrd1, and the putative helicase Sen1. Mol Cell Biol 16:6993–7003. doi: 10.1128/MCB.16.12.6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mitsuzawa H, Kanda E, Ishihama A. 2003. Rpb7 subunit of RNA polymerase II interacts with an RNA-binding protein involved in processing of transcripts. Nucleic Acids Res 31:4696–4701. doi: 10.1093/nar/gkg688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hashimoto R, Asano K, Tokashiki T, Onji Y, Hirose-yasumoto M, Takara R, Toyosato T, Yoshino A, Ikehata M, Ying L, Kumeda Y, Yokoyama K, Takahashi H. 2013. Mycotoxin production and genetic analysis of Aspergillus niger and related species including the Kuro-Koji mold. Mycotoxins 63:179–186. doi: 10.2520/myco.63.179 [DOI] [Google Scholar]
- 31. Yamada O, Machida M, Hosoyama A, Goto M, Takahashi T, Futagami T, Yamagata Y, Takeuchi M, Kobayashi T, Koike H, et al. 2016. Genome sequence of Aspergillus luchuensis NBRC 4314. DNA Res 23:507–515. doi: 10.1093/dnares/dsw032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Futagami T, Mori K, Yamashita A, Wada S, Kajiwara Y, Takashita H, Omori T, Takegawa K, Tashiro K, Kuhara S, Goto M. 2011. Genome sequence of the white koji mold Aspergillus kawachii IFO 4308, used for brewing the Japanese distilled spirit shochu. Eukaryot Cell 10:1586–1587. doi: 10.1128/EC.05224-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kadooka C, Goubaru S, Kubo S, Onoue M, Okutsu K, Yoshizaki Y, Takamine K, Goto M, Tamaki H, Futagami T. 2020. Analysis of the causative gene for the albino phenotype of the white koji fungus, Aspergillus luchuensis mut. kawachii NBRC 4308 (in Japanese with an English abstract). J Brew Soc Japan 115:369–377. doi: 10.6013/jbrewsocjapan.115.369 [DOI] [Google Scholar]
- 34. Futagami T. 2022. The white koji fungus Aspergillus luchuensis mut. kawachii. Biosci Biotechnol Biochem 86:574–584. doi: 10.1093/bbb/zbac033 [DOI] [PubMed] [Google Scholar]
- 35. Steinmetz EJ, Conrad NK, Brow DA, Corden JL. 2001. RNA-binding protein Nrd1 directs poly(A)-independent 3’-end formation of RNA polymerase II transcripts. Nature New Biol 413:327–331. doi: 10.1038/35095090 [DOI] [PubMed] [Google Scholar]
- 36. Arigo JT, Carroll KL, Ames JM, Corden JL. 2006. Regulation of yeast NRD1 expression by premature transcription termination. Mol Cell 21:641–651. doi: 10.1016/j.molcel.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 37. Keller NP. 2019. Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol 17:167–180. doi: 10.1038/s41579-018-0121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lind AL, Lim FY, Soukup AA, Keller NP, Rokas A. 2018. An LaeA- and BrlA-dependent cellular network governs tissue-specific secondary metabolism in the human pathogen Aspergillus fumigatus. mSphere 3:e00050-18. doi: 10.1128/mSphere.00050-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bok JW, Keller NP. 2004. LaeA, a regulator of secondary metabolism in Aspergillus spp. Euk Cell 3:527–535. doi: 10.1128/EC.3.2.527-535.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oda K, Kobayashi A, Ohashi S, Sano M. 2011. Aspergillus oryzae laeA regulates kojic acid synthesis genes. Biosci Biotechnol Biochem 75:1832–1834. doi: 10.1271/bbb.110235 [DOI] [PubMed] [Google Scholar]
- 41. Kawauchi M, Nishiura M, Iwashita K. 2013. Fungus-specific sirtuin HstD coordinates secondary metabolism and development through control of LaeA. Eukaryot Cell 12:1087–1096. doi: 10.1128/EC.00003-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vasiljeva L, Kim M, Terzi N, Soares LM, Buratowski S. 2008. Transcription termination and RNA degradation contribute to silencing of RNA polymerase II transcription within heterochromatin. Mol Cell 29:313–323. doi: 10.1016/j.molcel.2008.01.011 [DOI] [PubMed] [Google Scholar]
- 43. Parsa JY, Boudoukha S, Burke J, Homer C, Madhani HD. 2018. Polymerase pausing induced by sequence-specific RNA-binding protein drives heterochromatin assembly. Genes Dev 32:953–964. doi: 10.1101/gad.310136.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kadooka C, Nakamura E, Mori K, Okutsu K, Yoshizaki Y, Takamine K, Goto M, Tamaki H, Futagami T. 2020. LaeA controls citric acid production through regulation of the citrate exporter-encoding cexA gene in Aspergillus luchuensis mut. kawachii. Appl Environ Microbiol 86:e01950-19. doi: 10.1128/AEM.01950-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nishitani A, Hiramatsu K, Kadooka C, Mori K, Okutsu K, Yoshizaki Y, Takamine K, Tashiro K, Goto M, Tamaki H, Futagami T. 2023. Expression of heterochromatin protein 1 affects citric acid production in Aspergillus luchuensis mut. kawachii. J Biosci Bioeng 136:443–451. doi: 10.1016/j.jbiosc.2023.09.004 [DOI] [PubMed] [Google Scholar]
- 46. Miyamoto A, Kadooka C, Mori K, Tagawa Y, Okutsu K, Yoshizaki Y, Takamine K, Goto M, Tamaki H, Futagami T. 2020. Sirtuin SirD is involved in α-amylase activity and citric acid production in Aspergillus luchuensis mut. kawachii during a solid-state fermentation process. J Biosci Bioeng 129:454–466. doi: 10.1016/j.jbiosc.2019.11.004 [DOI] [PubMed] [Google Scholar]
- 47. Itoh E, Odakura R, Oinuma KI, Shimizu M, Masuo S, Takaya N. 2017. Sirtuin E is a fungal global transcriptional regulator that determines the transition from the primary growth to the stationary phase. J Biol Chem 292:11043–11054. doi: 10.1074/jbc.M116.753772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wassano NS, da Silva GB, Reis AH, A Gerhardt J, Antoniel EP, Akiyama D, Rezende CP, Neves LX, Vasconcelos EJR, de Figueiredo FL, Almeida F, de Castro PA, Pinzan CF, Goldman GH, Paes Leme AF, Fill TP, Moretti NS, Damasio A. 2024. Sirtuin E deacetylase is required for full virulence of Aspergillus fumigatus. Commun Biol 7:704. doi: 10.1038/s42003-024-06383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Niu J, Arentshorst M, Nair PDS, Dai Z, Baker SE, Frisvad JC, Nielsen KF, Punt PJ, Ram AFJ. 2016. Identification of a classical mutant in the industrial host Aspergillus niger by systems genetics: LaeA is required for citric acid production and regulates the formation of some secondary metabolites . G3 Genes Genomes Genetics 6:193–204. doi: 10.1534/g3.115.024067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Linde T, Zoglowek M, Lübeck M, Frisvad JC, Lübeck PS. 2016. The global regulator LaeA controls production of citric acid and endoglucanases in Aspergillus carbonarius. J Ind Microbiol Biotechnol 43:1139–1147. doi: 10.1007/s10295-016-1781-3 [DOI] [PubMed] [Google Scholar]
- 51. Kadooka C, Onitsuka S, Uzawa M, Tashiro S, Kajiwara Y, Takashita H, Okutsu K, Yoshizaki Y, Takamine K, Goto M, Tamaki H, Futagami T. 2016. Marker recycling system using the sC gene in the white koji mold, Aspergillus luchuensis mut. kawachii. J Gen Appl Microbiol 62:160–163. doi: 10.2323/jgam.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 52. Mizutani O, Kudo Y, Saito A, Matsuura T, Inoue H, Abe K, Gomi K. 2008. A defect of LigD (human Lig4 homolog) for nonhomologous end joining significantly improves efficiency of gene-targeting in Aspergillus oryzae. Fungal Genet Biol 45:878–889. doi: 10.1016/j.fgb.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 53. Nakamura E, Kadooka C, Okutsu K, Yoshizaki Y, Takamine K, Goto M, Tamaki H, Futagami T. 2021. Citrate exporter enhances both extracellular and intracellular citric acid accumulation in the koji fungi Aspergillus luchuensis mut. kawachii and Aspergillus oryzae. J Biosci Bioeng 131:68–76. doi: 10.1016/j.jbiosc.2020.09.002 [DOI] [PubMed] [Google Scholar]
- 54. Barratt RW, Johnson GB, Ogata WN. 1965. Wild-type and mutant stocks of Aspergillus nidulans. Genetics 52:233–246. doi: 10.1093/genetics/52.1.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meyer V, Wanka F, van Gent J, Arentshorst M, van den Hondel CAMJJ, Ram AFJ. 2011. Fungal gene expression on demand: an inducible, tunable, and metabolism-independent expression system for Aspergillus niger. Appl Environ Microbiol 77:2975–2983. doi: 10.1128/AEM.02740-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kubodera T, Yamashita N, Nishimura A. 2002. Transformation of Aspergillus sp. and Trichoderma reesei using the pyrithiamine resistance gene (ptrA) of Aspergillus oryzae. Biosci Biotechnol Biochem 66:404–406. doi: 10.1271/bbb.66.404 [DOI] [PubMed] [Google Scholar]
- 57. Liu H-L, Osmani AH, Ukil L, Son S, Markossian S, Shen K-F, Govindaraghavan M, Varadaraj A, Hashmi SB, De Souza CP, Osmani SA. 2010. Single-step affinity purification for fungal proteomics. Eukaryot Cell 9:831–833. doi: 10.1128/EC.00032-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang L, Ukil L, Osmani A, Nahm F, Davies J, De Souza CPC, Dou X, Perez-Balaguer A, Osmani SA. 2004. Rapid production of gene replacement constructs and generation of a green fluorescent protein-tagged centromeric marker in Aspergillus nidulans. Eukaryot Cell 3:1359–1362. doi: 10.1128/EC.3.5.1359-1362.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10. doi: 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- 61. Dobin A, Gingeras TR. 2016. Optimizing RNA-Seq mapping with STAR. Methods Mol Biol 1415:245–262. doi: 10.1007/978-1-4939-3572-7_13 [DOI] [PubMed] [Google Scholar]
- 62. Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. doi: 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- 63. Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Si Y, Liu P, Li P, Brutnell TP. 2014. Model-based clustering for RNA-seq data. Bioinformatics 30:197–205. doi: 10.1093/bioinformatics/btt632 [DOI] [PubMed] [Google Scholar]
- 65. Cerqueira GC, Arnaud MB, Inglis DO, Skrzypek MS, Binkley G, Simison M, Miyasato SR, Binkley J, Orvis J, Shah P, Wymore F, Sherlock G, Wortman JR. 2014. The Aspergillus Genome Database: multispecies curation and incorporation of RNA-Seq data to improve structural gene annotations. Nucleic Acids Res 42:D705–D710. doi: 10.1093/nar/gkt1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hong SB, Lee M, Kim DH, Varga J, Frisvad JC, Perrone G, Gomi K, Yamada O, Machida M, Houbraken J, Samson RA. 2013. Aspergillus luchuensis, an industrially important black Aspergillus in East Asia. PLoS ONE 8:e63769. doi: 10.1371/journal.pone.0063769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hong SB, Yamada O, Samson RA. 2014. Taxonomic re-evaluation of black koji molds. Appl Microbiol Biotechnol 98:555–561. doi: 10.1007/s00253-013-5332-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differential analysis of RNA-seq and RIP-seq.
Fig. S1 to S8; Tables S1 and S2.
Data Availability Statement
The data obtained from the RNA-seq and RIP-seq analyses in this study were deposited in the Gene Expression Omnibus under accession number GSE164392.