Abstract
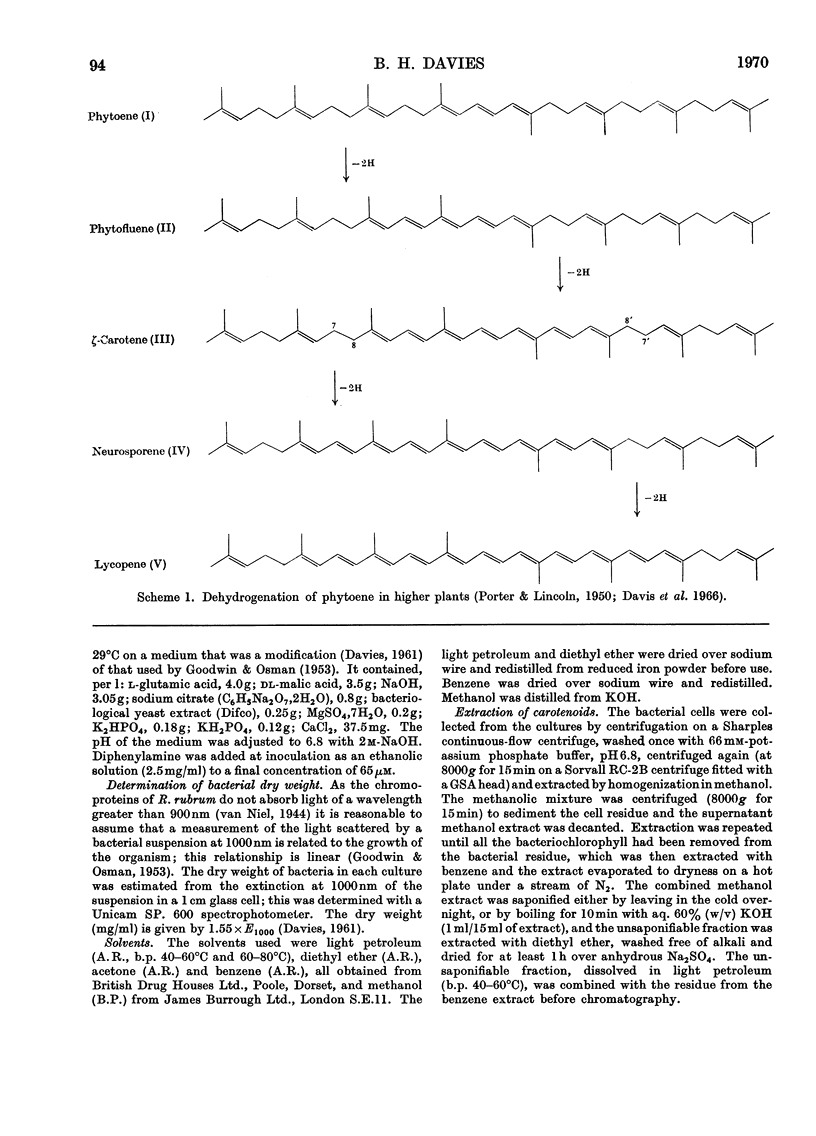
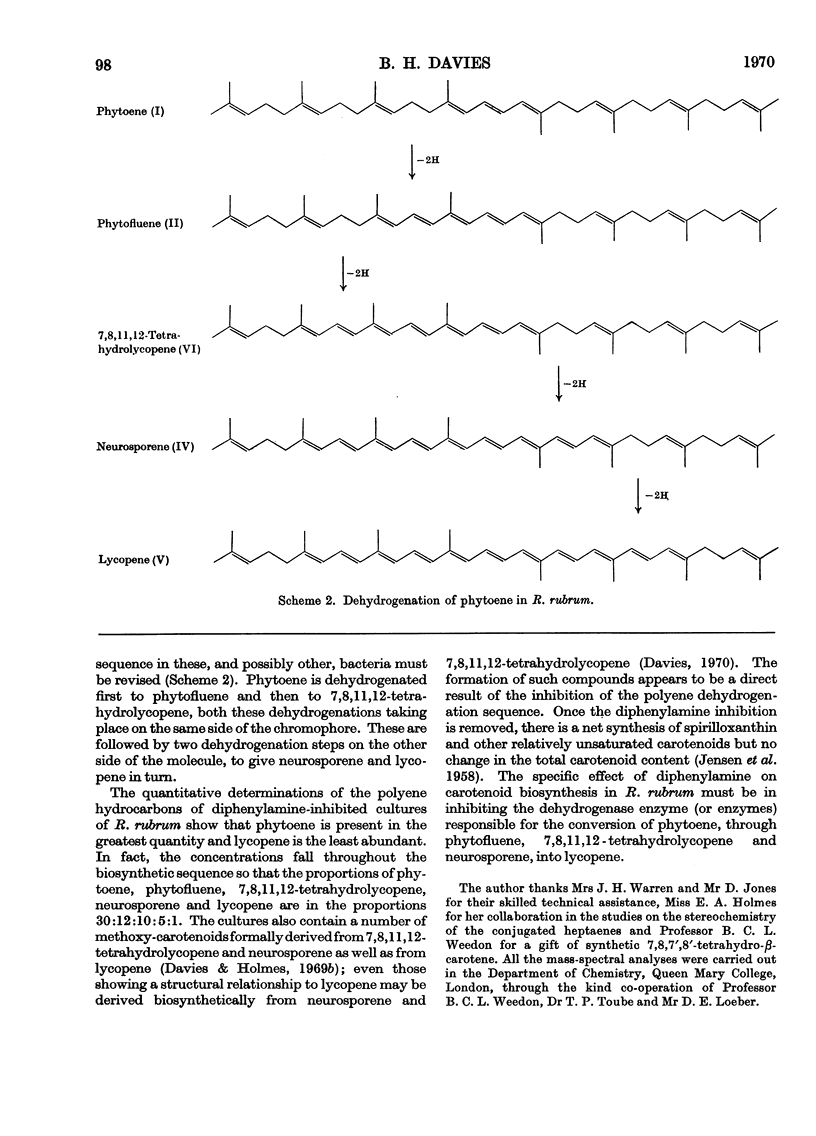
Differences between the absorption spectra of ζ-carotene (7,8,7′,8′-tetrahydrolycopene) and the corresponding conjugated heptaene from diphenylamine-inhibited cultures of Rhodospirillum rubrum have been rationalized by the identification of the latter compound as the unsymmetrical isomer, 7,8,11,12-tetrahydrolycopene. The structures of the other conjugated polyene hydrocarbons, phytoene, phytofluene and neurosporene, have been confirmed and a novel pathway for the dehydrogenation of phytoene to lycopene in these bacteria is described.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chichester C. O. The biosynthesis of carotenoids. Pure Appl Chem. 1967;14(2):215–226. doi: 10.1351/pac196714020215. [DOI] [PubMed] [Google Scholar]
- Davies B. H. Alternative pathways of spirilloxanthin biosynthesis in Rhodospirillum rubrum. Biochem J. 1970 Jan;116(1):101–110. doi: 10.1042/bj1160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B. H., Holmes E. A. New pathways of methoxy carotenoid formation in Rhodospirillum rubrum. Biochem J. 1969 Jul;113(3):34P–35P. doi: 10.1042/bj1130034pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W., OSMAN H. G. Studies in carotenogenesis. 10. Spirilloxanthin synthesis by washed cells of Rhodospirillum rubrum. Biochem J. 1954 Feb;56(2):222–230. doi: 10.1042/bj0560222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W., OSMAN H. G. Studies in carotenogenesis. 9. General cultural conditions controlling carotenoid (spirilloxanthin) synthesis in the photosynthetic bacterium Rhodospirillum rubrum. Biochem J. 1953 Mar;53(4):541–546. doi: 10.1042/bj0530541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. III. Identification of the minor polyene components of the fungus Phycomyces blakesleeanus and a study of their synthesis under various cultural conditions. Biochem J. 1952 Feb;50(4):550–558. doi: 10.1042/bj0500550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTFREUND H., STURTEVANT J. M. Steps in the oxidation of xanthine to uric acid catalysed by milk xanthine oxidase. Biochem J. 1959 Sep;73:1–6. doi: 10.1042/bj0730001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN S. L., COHEN-BAZIRE G., NAKAYAMA T. O., STANIER R. Y. The path of carotenoid synthesis in a photosynthetic bacterium. Biochim Biophys Acta. 1958 Sep;29(3):477–498. doi: 10.1016/0006-3002(58)90003-9. [DOI] [PubMed] [Google Scholar]
- JENSEN S. L., COHEN-BAZIRE G., STANIER R. Y. Biosynthesis of carotenoids in purple bacteria: a reevaluation based on considerations of chemical structure. Nature. 1961 Dec 23;192:1168–1172. doi: 10.1038/1921168a0. [DOI] [PubMed] [Google Scholar]
- PORTER J. W., LINCOLN R. E. Lycopersicon selections containing a high content of carotenes and colorless polyenes; the mechanism of carotene biosynthesis. Arch Biochem. 1950 Jul;27(2):390–403. [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]


