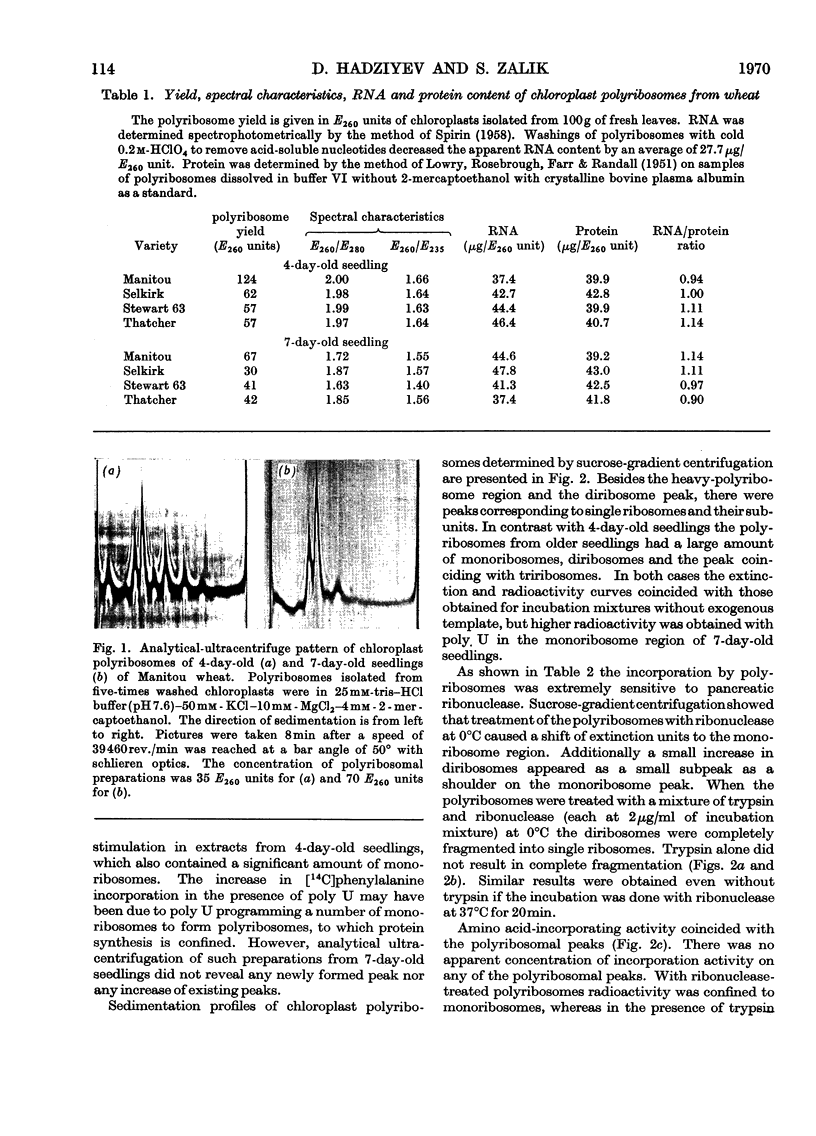
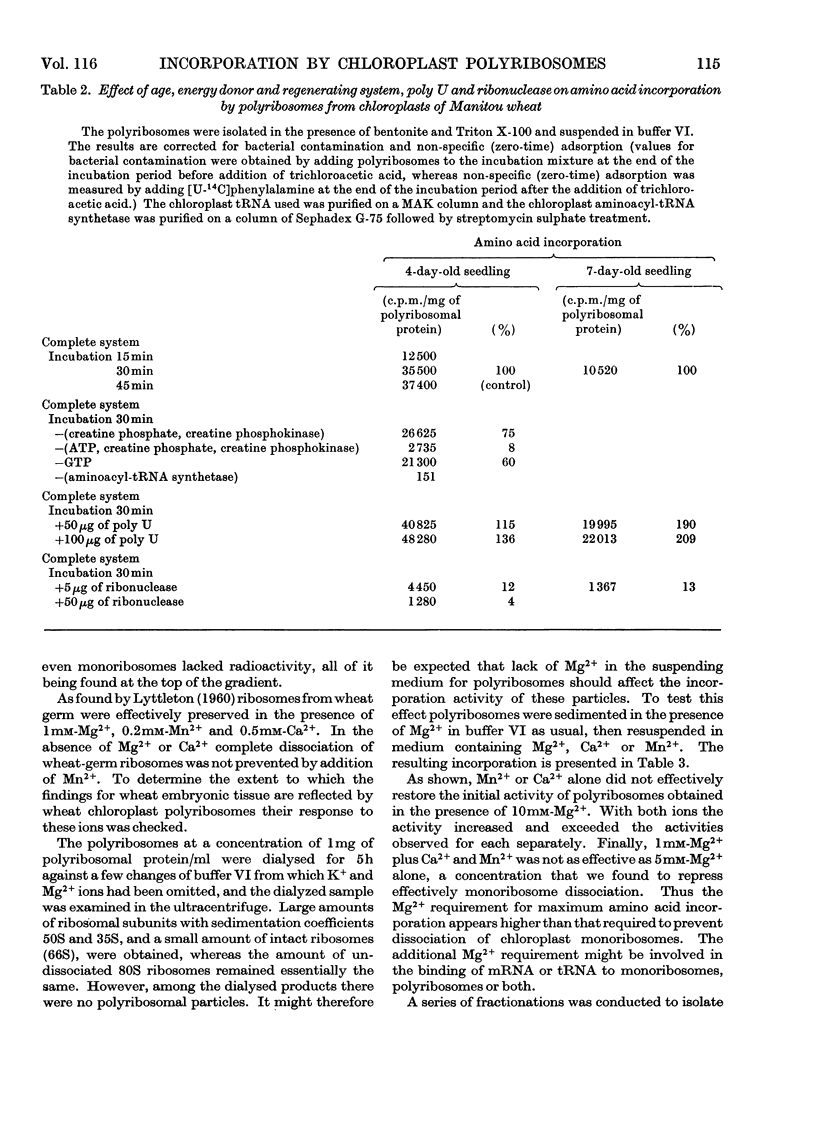
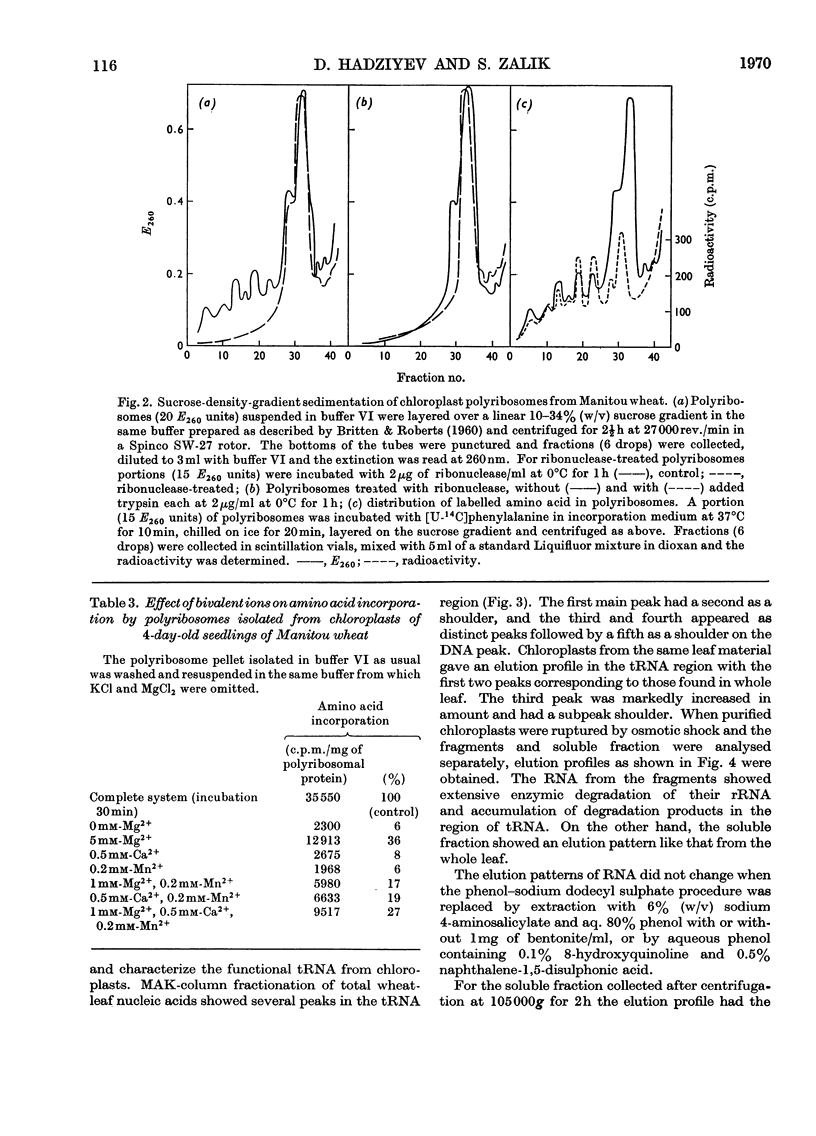
Abstract
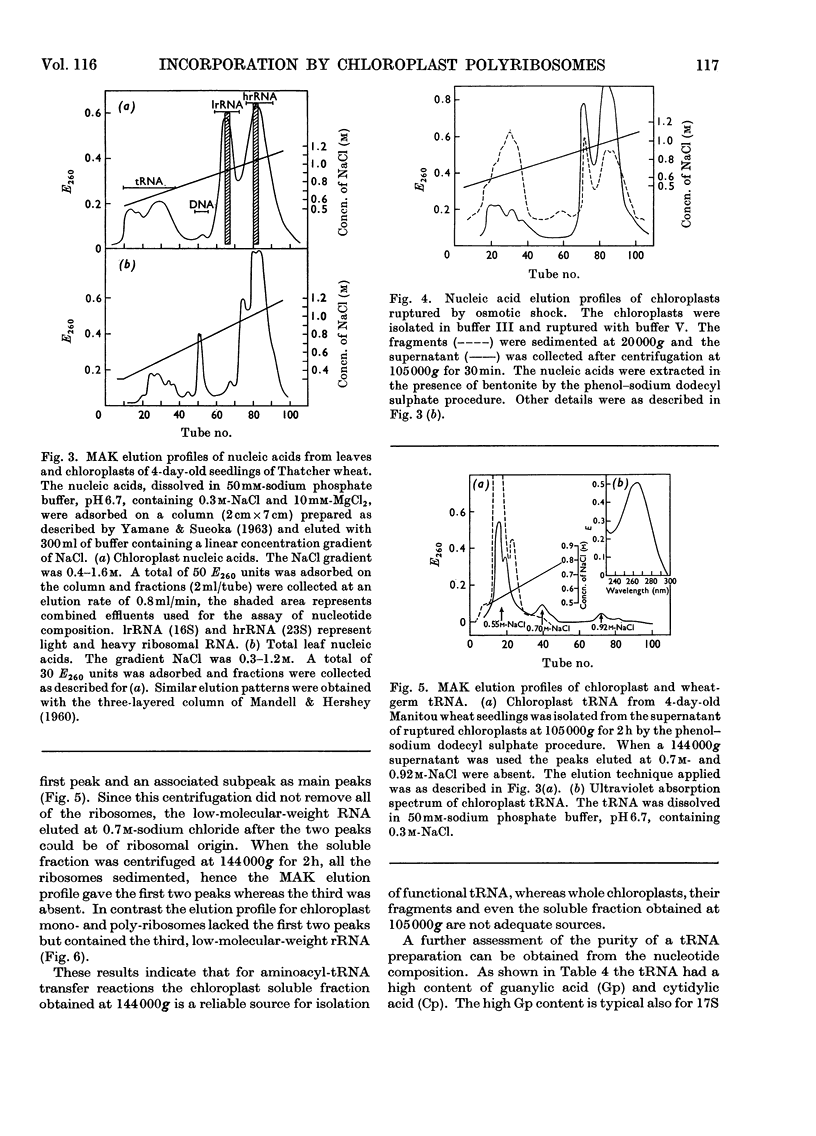
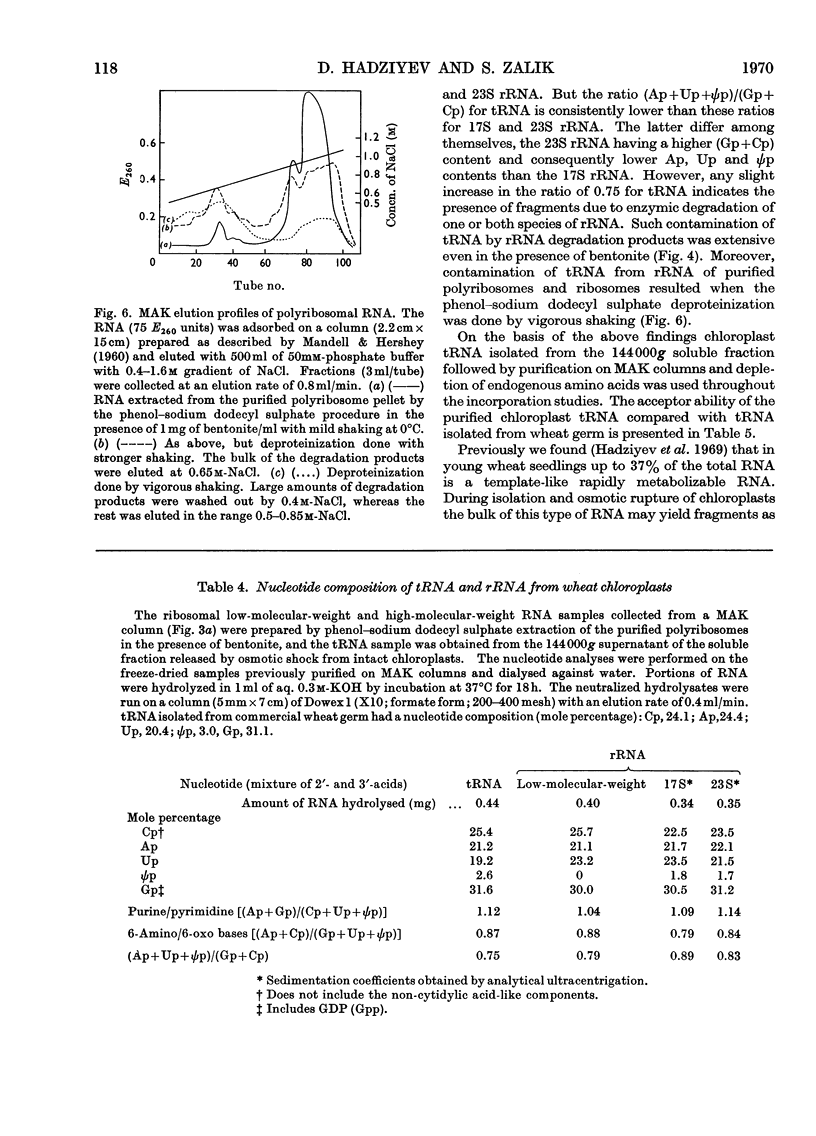
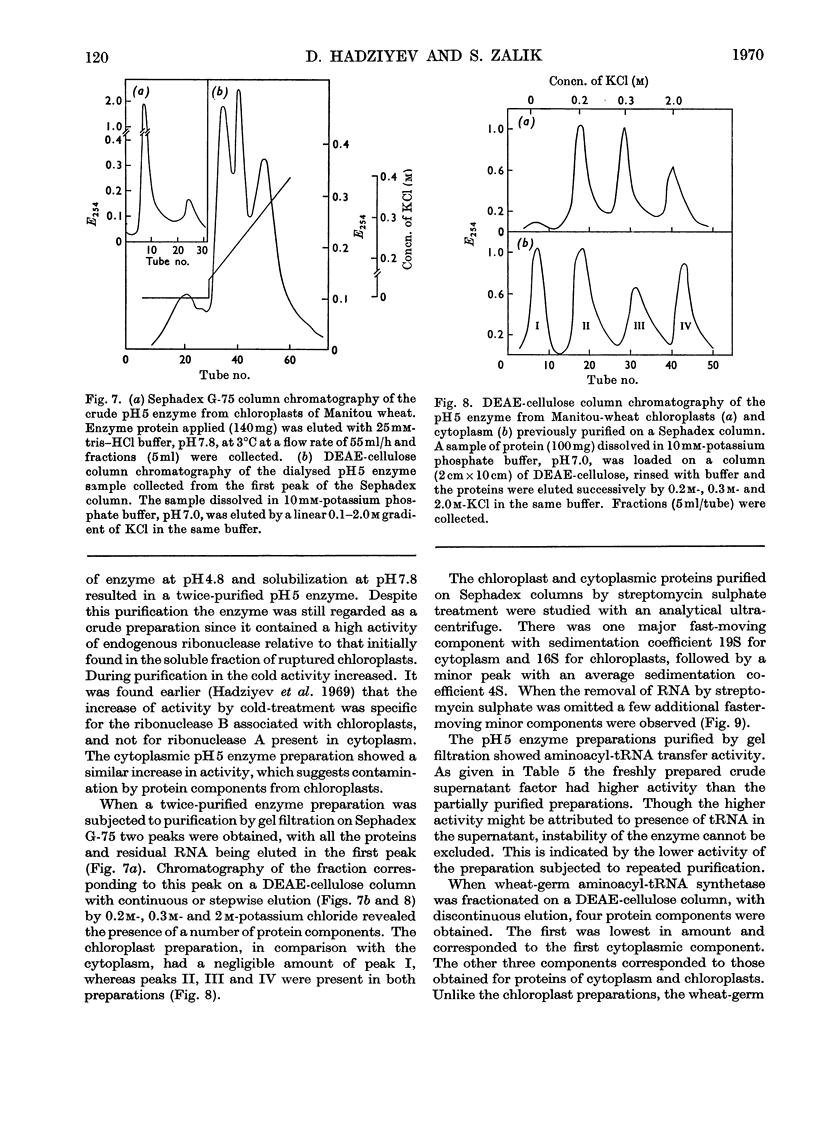
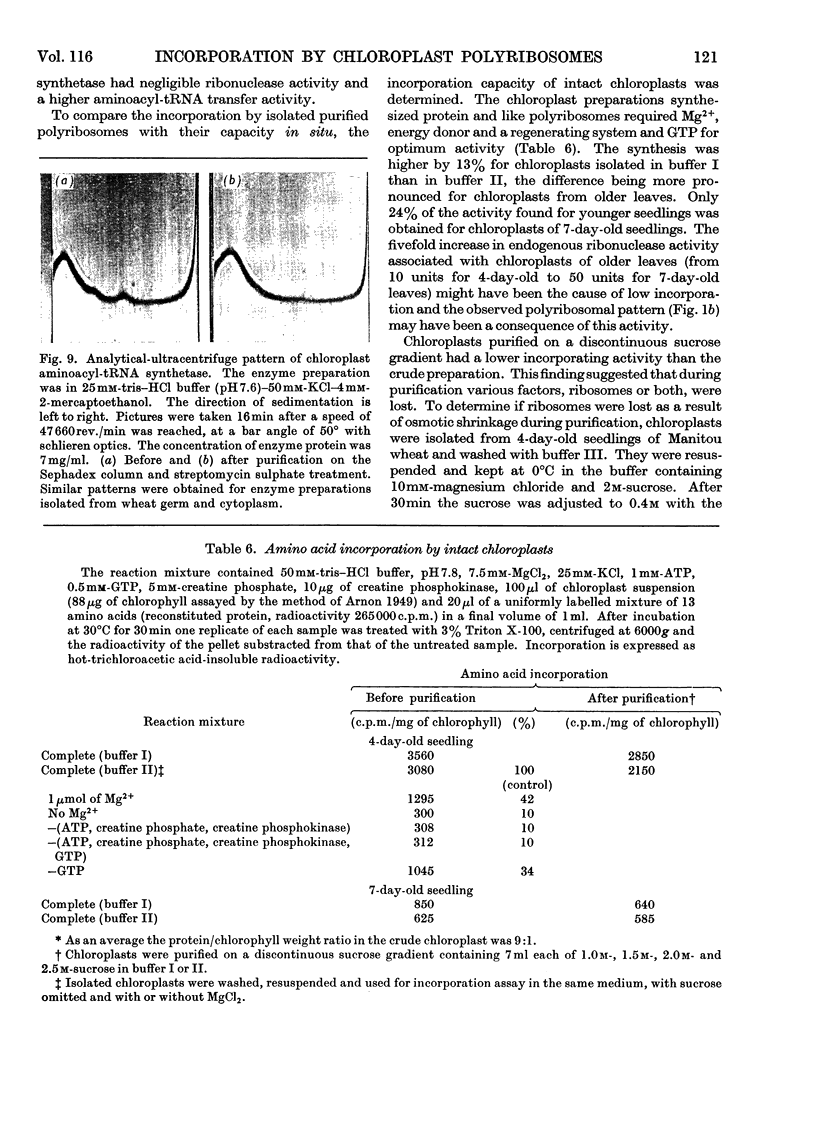
Sucrose-gradient and analytical ultracentrifugation showed that chloroplast polyribosomes from 4-day-old seedlings had mono-, di-, tri-, tetra- and traces of penta-ribosomes, in contrast with those from 7-day-old seedlings in which only the mono-, di- and traces of tri-ribosomes were present. Without Mg2+ the polyribosomes dissociated into ribosomal subunits. The rate of l-[U-14C]phenylalanine incorporation was threefold greater for preparations from 4- than from 7-day-old seedlings. Incorporation by the latter was stimulated by polyuridylic acid. The rates of incorporation were similar whether the reaction mixture contained chloroplast or wheat-germ transfer RNA and amino acid synthetases purified on methylated albumin-on-kieselguhr and Sephadex G-75 columns respectively. The cofactor requirement was the same as for isolated intact chloroplasts. Osmotic rupture of chloroplasts with and without Triton X-100 revealed the presence of free and bound ribosomes. Free single ribosomes isolated by osmotic shrinkage or prepared by pancreatic ribonuclease digestion of chloroplast polyribosomes had negligible incorporation activity. This activity was increased by washing or by polyuridylic acid, but was still only a fraction of that given by polyribosomes. A comparison of incorporation activity of chloroplast polyribosomes with those from the surrounding cytoplasm showed the former to be 20 times more active.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende J. E., Bravo M. Amino acid incorporation and aminoacyl transfer in a wheat embryo system. J Biol Chem. 1966 Dec 25;241(24):5813–5818. [PubMed] [Google Scholar]
- Bamji M. S., Jagendorf A. T. Amino Acid incorporation by wheat chloroplasts. Plant Physiol. 1966 May;41(5):764–770. doi: 10.1104/pp.41.5.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K., Francki R. I., Wildman S. G. Protein synthesis by cell-free extracts of tobacco leaves. 3. Comparison of the physical properties and protein synthesizing activities of 70 s chloroplast and 80 s cytoplasmic ribosomes. J Mol Biol. 1966 Jun;17(2):470–487. doi: 10.1016/s0022-2836(66)80157-2. [DOI] [PubMed] [Google Scholar]
- Brentani R., Brentani M., Raw I., Cunha J. L., Wrotschincky N. The effect of ribonuclease on rat-liver ribosomes. Biochem J. 1968 Jan;106(1):263–266. doi: 10.1042/bj1060263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLITZ D. G., DEKKER C. A. THE PURIFICATION AND PROPERTIES OF RIBONUCLEIC ACID FROM WHEAT GERM. Biochemistry. 1963 Nov-Dec;2:1185–1192. doi: 10.1021/bi00906a002. [DOI] [PubMed] [Google Scholar]
- Hadziyev D., Mehta S. L., Zalik S. Nucleic acids and ribonucleases of wheat leaves and chloroplasts. Can J Biochem. 1969 Mar;47(3):273–282. doi: 10.1139/o69-042. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leaver C. J., Key J. L. Polyribosome formation and RNA synthesis during aging of carrot-root tissue. Proc Natl Acad Sci U S A. 1967 May;57(5):1338–1344. doi: 10.1073/pnas.57.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Marcus A., Feeley J. Ribosome activation and polysome formation in vitro: requirement for ATP. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1770–1777. doi: 10.1073/pnas.56.6.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A., Feeley J., Volcani T. Protein Synthesis in Imbibed Seeds III. Kinetics of Amino Acid Incorporation Ribosome Activation, and Polysome Formation. Plant Physiol. 1966 Sep;41(7):1167–1172. doi: 10.1104/pp.41.7.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S. L., Hadziyev D., Zalik S. Chloroplast and cytoplasmic polysomes and ribosomal RNA from wheat. Biochim Biophys Acta. 1968 Dec 17;169(2):381–386. doi: 10.1016/0005-2787(68)90046-4. [DOI] [PubMed] [Google Scholar]
- Melik-Sarkisian S. S., Kuznetsova M. D., Avdeeva T. A., Sisakian N. M. Fiziko-khimicheskie svoistva i fermentativnaia aktivnost' rastvorimykh belkov lista. Biokhimiia. 1967 Jan-Feb;32(1):189–197. [PubMed] [Google Scholar]
- Parenti-Rosina R., Eisenstadt A., Eisenstadt J. M. Isolation of protein initiation factors from 30S ribosomal subunits. Nature. 1969 Jan 25;221(5178):363–365. doi: 10.1038/221363a0. [DOI] [PubMed] [Google Scholar]
- Raacke I. D., Fiala J. Effects of 'pre-incubation' on the distribution of different enzymes in extracts of Escherichia coli. Nature. 1965 Mar 13;205(976):1072–1074. doi: 10.1038/2051072a0. [DOI] [PubMed] [Google Scholar]
- SPENCER D., WILDMAN S. G. THE INCORPORATION OF AMINO ACIDS INTO PROTEIN BY CELL-FREE EXTRACTS FROM TOBACCO LEAVES. Biochemistry. 1964 Jul;3:954–959. doi: 10.1021/bi00895a019. [DOI] [PubMed] [Google Scholar]
- TAKANAMI M., ZUBAY G. AN ESTIMATE OF THE SIZE OF THE RIBOSOMAL SITE FOR MESSENGER RNA BINDING. Proc Natl Acad Sci U S A. 1964 May;51:834–839. doi: 10.1073/pnas.51.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S., Sypherd P. S. Changes in soluble RNA and ribonuclease activity during germination of wheat. Plant Physiol. 1968 Aug;43(8):1221–1226. doi: 10.1104/pp.43.8.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]