Abstract
1. Homogenates of guinea-pig polymorphonuclear leucocytes were separated by differential centrifugation into six particulate fractions and a soluble fraction. 2. The distributions in these fractions of protein, DNA, succinate dehydrogenase, β-glucuronidase, peroxidase, alkaline phosphatase, acid phosphatase (against p-nitrophenyl phosphate and β-glycerophosphate), cathepsin, and catalase were compared. 3. Almost all of the DNA sedimented in the first two pellets, indicating that the nuclei were relatively intact. 4. The four hydrolases and peroxidase showed different distribution patterns, although these activities were previously reported to be localized mainly in the single `granule' fraction isolated from leucocytes. 5. The particles containing peroxidase, acid phosphatase and alkaline phosphatase all exhibited latency. Maximum activity for each enzyme was obtained at roughly similar concentrations of Triton X-100. 6. The acid phosphatase of these cells was distributed between two populations of particles that differed in both sedimentation characteristics and density. The acid phosphatase(s) of the two populations showed slightly different substrate specificities. This bimodal distribution was not an artifact of the procedure used to elicit the cells. 7. Catalase was recovered almost entirely in the soluble fraction and showed no latency in freshly prepared homogenates. No urate oxidase was detected. 8. We conclude that the `granule' fraction of the polymorphonuclear leucocyte, as isolated by previous workers, contains at least three, probably more, populations of particles with different enzyme contents, and that these cells probably do not contain peroxisomes.
Full text
PDF


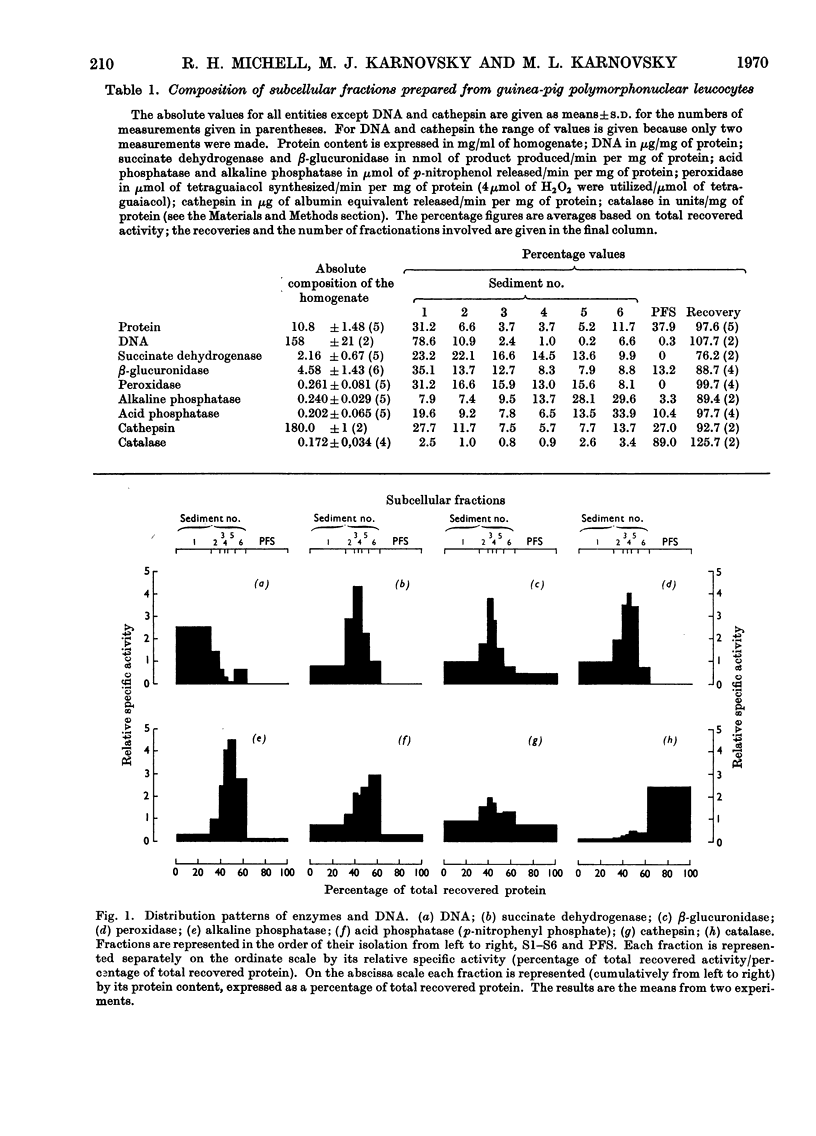
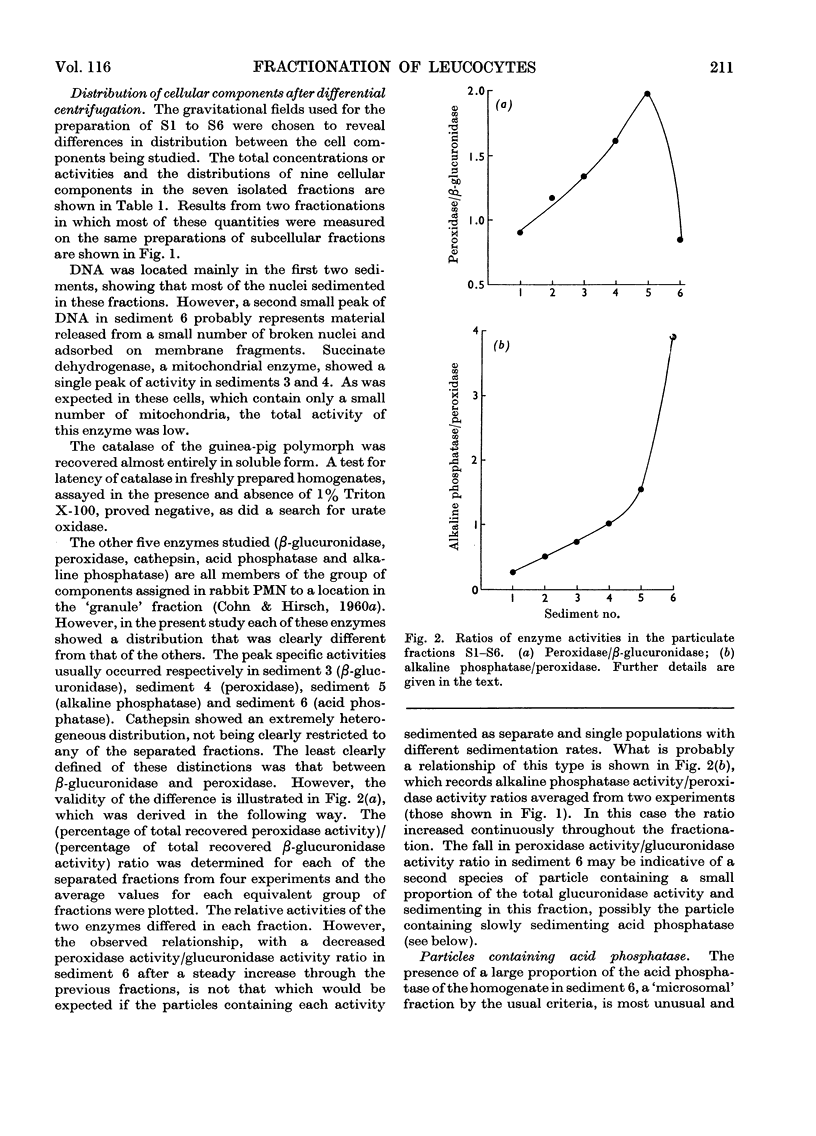
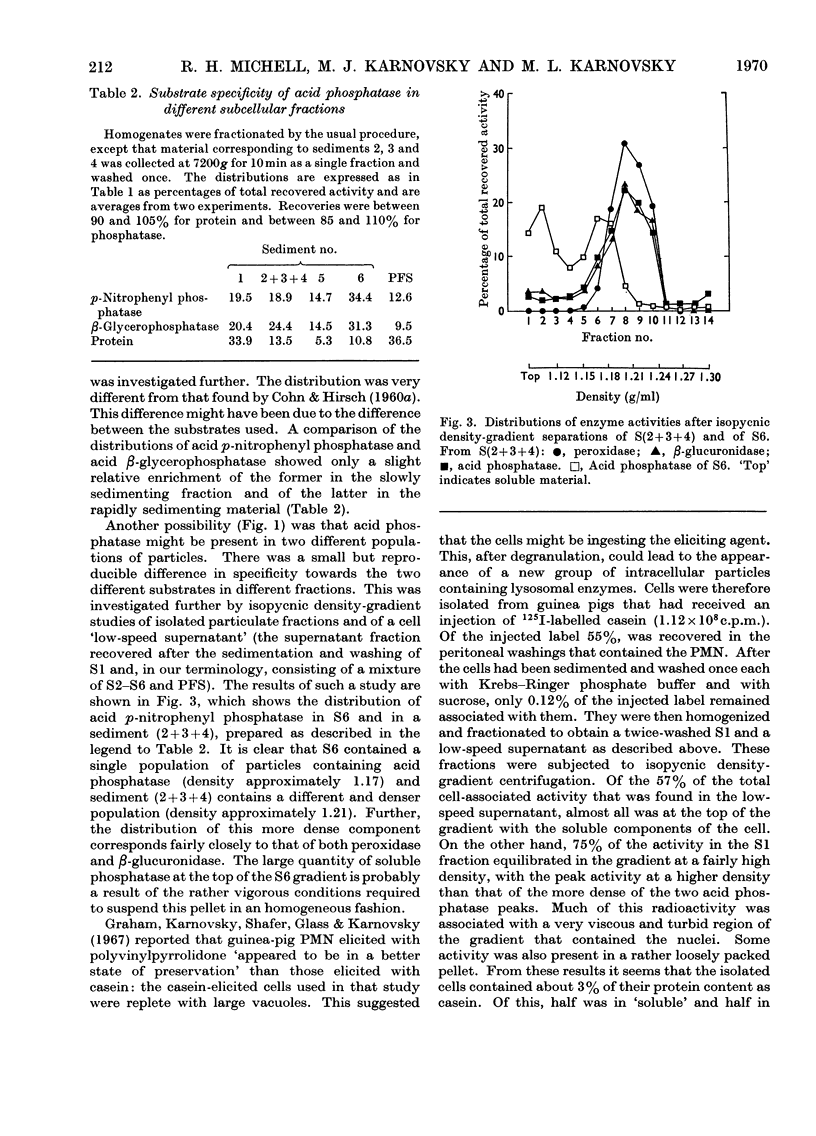
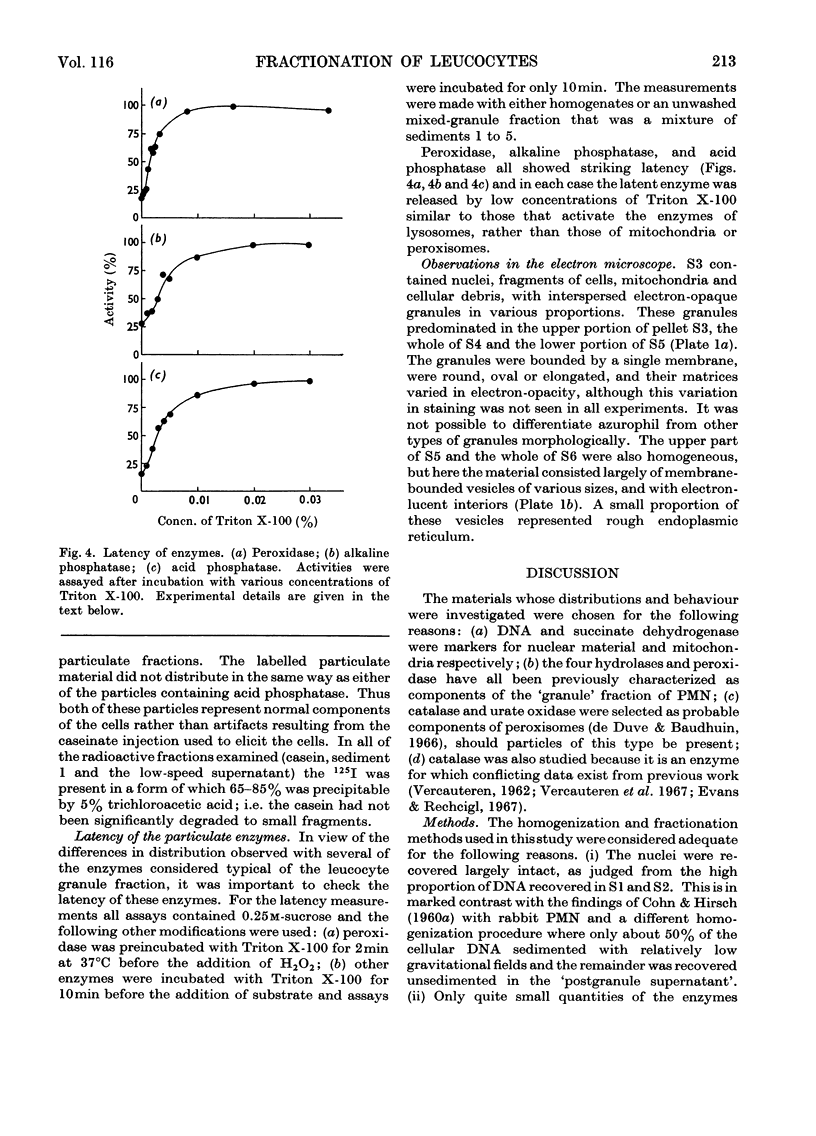

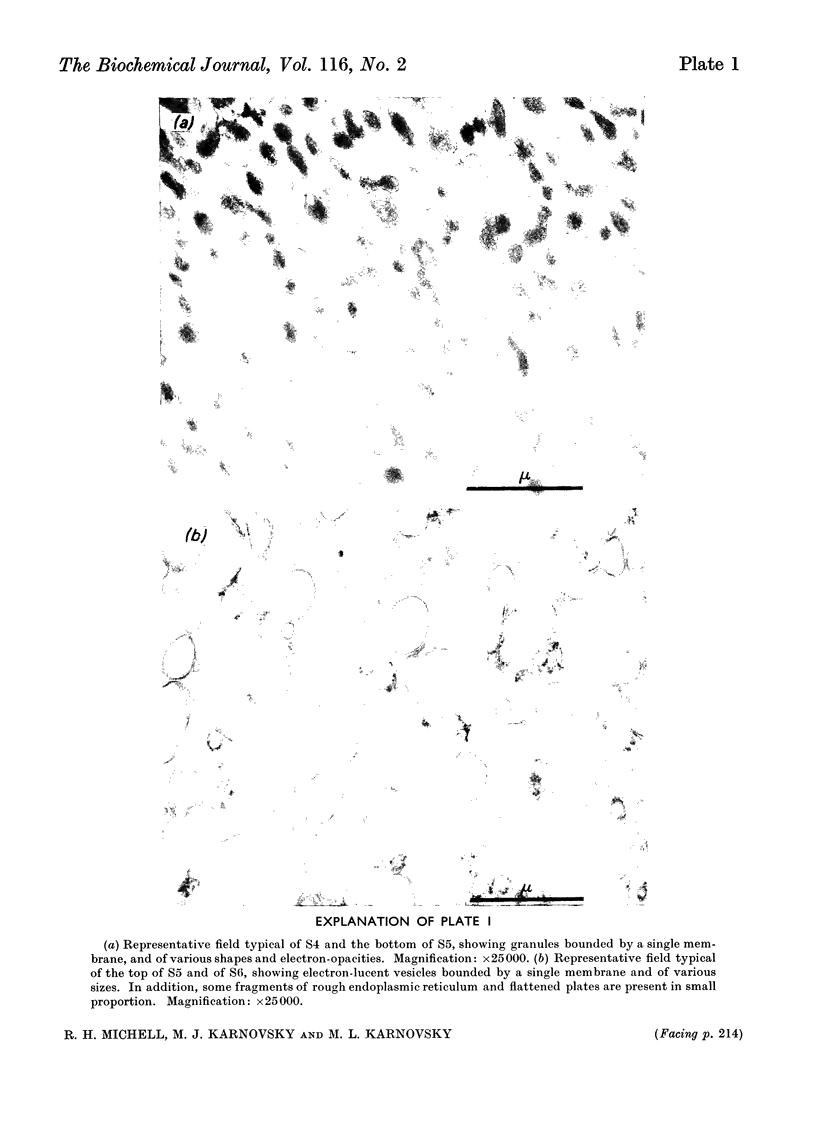


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Hirsch J. G., De Duve C. Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J Cell Biol. 1969 Feb;40(2):529–541. doi: 10.1083/jcb.40.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. I. Histochemical staining of bone marrow smears. J Cell Biol. 1968 Nov;39(2):286–298. doi: 10.1083/jcb.39.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. II. Cytochemistry and electron microscopy of bone marrow cells. J Cell Biol. 1968 Nov;39(2):299–317. doi: 10.1083/jcb.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Origin of granules in polymorphonuclear leukocytes. Two types derived from opposite faces of the Golgi complex in developing granulocytes. J Cell Biol. 1966 Feb;28(2):277–301. doi: 10.1083/jcb.28.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Jacques P., Baudhuin P., Sellinger O. Z., Berthet J., De Duve C. Tissue fractionation studies. 18. Resolution of mitochondrial fractions from rat liver into three distinct populations of cytoplasmic particles by means of density equilibration in various gradients. Biochem J. 1964 Jul;92(1):184–205. doi: 10.1042/bj0920184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The influence of phagocytosis on the intracellular distribution of granule-associated components of polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:1015–1022. doi: 10.1084/jem.112.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The isolation and properties of the specific cytoplasmic granules of rabbit polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:983–1004. doi: 10.1084/jem.112.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BURSTONE M. S., WALTER P. C., KINSLEY J. W. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J Cell Biol. 1963 Jun;17:465–486. doi: 10.1083/jcb.17.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- De Duve C. Principles of tissue fractionation. J Theor Biol. 1964 Jan;6(1):33–59. doi: 10.1016/0022-5193(64)90065-7. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Kitani T. [Electron microscopic studies on peroxidase and acid phosphatase reaction in human leukocytes (in normal and leukemic cells and on phagocytosis)]. Nihon Ketsueki Gakkai Zasshi. 1966 Aug;29(4):554–570. [PubMed] [Google Scholar]
- Evans W. H., Rechcigl M., Jr Factors influencing myeloperoxidase and catalase activities in polymorphonuclear leukocytes. Biochim Biophys Acta. 1967 Oct 9;148(1):243–250. doi: 10.1016/0304-4165(67)90299-1. [DOI] [PubMed] [Google Scholar]
- GEORGE P. Intermediate compound formation with peroxidase and strong oxidizing agents. J Biol Chem. 1953 Mar;201(1):413–426. [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOFFMAN W., NEWILL V. A. GENERALIZATION OF EPIDEMIC THEORY. AN APPLICATION TO THE TRANSMISSION OF IDEAS. Nature. 1964 Oct 17;204:225–228. doi: 10.1038/204225a0. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J., Shafer A. W., Glass E. A., Karnovsky M. L. Metabolic and morphological observations on the effect of surface-active agents of leukocytes. J Cell Biol. 1967 Mar;32(3):629–647. doi: 10.1083/jcb.32.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCHHORN R., WEISSMANN G. ISOLATION AND PROPERTIES OF HUMAN LEUKOCYTE LYSOSOMES IN VITRO. Proc Soc Exp Biol Med. 1965 May;119:36–39. doi: 10.3181/00379727-119-30091. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G., COHN Z. A. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J Exp Med. 1960 Dec 1;112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORN R. G., SPICER S. S., WETZEL B. K. PHAGOCYTOSIS OF BACTERIA BY HETEROPHIL LEUKOCYTES: ACID AND ALKALINE PHOSPHATASE CYTOCHEMISTRY. Am J Pathol. 1964 Aug;45:327–335. [PMC free article] [PubMed] [Google Scholar]
- KUFF E. L., HOGEBOOM G. H., DALTON A. J. Centrifugal, biochemical, and electron microscopic analysis of cytoplasmic particulates in liver homogenates. J Biophys Biochem Cytol. 1956 Jan 25;2(1):33–54. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michell R. H., Pancake S. J., Noseworthy J., Karnovsky M. L. Measurement of rates of phagocytosis: the use of cellular monolayers. J Cell Biol. 1969 Jan;40(1):216–224. doi: 10.1083/jcb.40.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEASE D. C. An electron microscopic study of red bone marrow. Blood. 1956 Jun;11(6):501–526. [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTEOUS J. W., CLARK B. THE ISOLATION AND CHARACTERIZATION OF SUBCELLULAR COMPONENTS OF THE EPITHELIAL CELLS OF RABBIT SMALL INTESTINE. Biochem J. 1965 Jul;96:159–171. doi: 10.1042/bj0960159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzansky J. J., Patterson R. Subcellular distribution of histamine in human leucocytes. Proc Soc Exp Biol Med. 1967 Jan;124(1):56–59. doi: 10.3181/00379727-124-31665. [DOI] [PubMed] [Google Scholar]
- SCHULTZ J., KAMINKER K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962 Mar;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- Schultz J., Corlin R., Oddi F., Kaminker K., Jones W. Myeloperoxidase of the leucocyte of normal human blood. 3. Isolation of the peroxidase granule. Arch Biochem Biophys. 1965 Jul;111(1):73–79. doi: 10.1016/0003-9861(65)90324-3. [DOI] [PubMed] [Google Scholar]
- Takikawa K., Ohta H. [On the nature of neutrophilic granules]. Nihon Ketsueki Gakkai Zasshi. 1966 Aug;29(4):571–577. [PubMed] [Google Scholar]
- VERCAUTEREN R. E. CONTRIBUTION TO THE PROBLEM OF THE ENZYMIC EQUIPMENT OF THE SPECIFIC GRANULES OF LEUCOCYTES. Enzymologia. 1964 Nov 15;27:369–381. [PubMed] [Google Scholar]
- VERCAUTEREN R. E. Oxidoreductases of leucocytes. II. Evidence for particulate bound catalase and peroxidase in leucocyte homogenates. Enzymologia. 1962 Jan 2;24:37–48. [PubMed] [Google Scholar]
- Vercauteren R. E., Roels-de Schrijver M. P., Decleir W. Further studies on the specific granules of leucocytes. Enzymologia. 1967 Nov 30;33(5):279–298. [PubMed] [Google Scholar]
- WEISSMANN G., BECHER B., THOMAS L. STUDIES ON LYSOSOMES. V. THE EFFECTS OF STREPTOLYSINS AND OTHER HEMOLYTIC AGENTS ON ISOLATED LEUCOCYTE GRANULES. J Cell Biol. 1964 Jul;22:115–126. doi: 10.1083/jcb.22.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODIN A. M., FRENCH J. E., MARCHESI V. T. Morphological changes associated with the extrusion of protein induced in the polymorphonuclear leucocyte by staphylococcal leucocidin. Biochem J. 1963 Jun;87:567–571. doi: 10.1042/bj0870567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODIN A. M. The extrusion of protein from the rabbit polymorphonuclear leucocyte treated with staphylococcal leucocidin. Biochem J. 1962 Jan;82:9–15. doi: 10.1042/bj0820009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe I., Donahue S., Hoggatt N. Method for electron microscopic studies of circulating human leukocytes and observations on their fine structure. J Ultrastruct Res. 1967 Oct 31;20(5):366–382. doi: 10.1016/s0022-5320(67)80106-0. [DOI] [PubMed] [Google Scholar]
- Wetzel B. K., Horn R. G., Spicer S. S. Fine structural studies on the development of heterophil, eosinophil, and basophil granulocytes in rabbits. Lab Invest. 1967 Mar;16(3):349–382. [PubMed] [Google Scholar]
- Wetzel B. K., Spicer S. S., Horn R. G. Fine structural localization of acid and alkaline phosphatases in cells of rabbit blood and bone marrow. J Histochem Cytochem. 1967 Jun;15(6):311–334. doi: 10.1177/15.6.311. [DOI] [PubMed] [Google Scholar]
- Woodin A. M., Wieneke A. A. The participation of calcium, adenosine triphosphate and adenosine triphosphatase in the extrusion of the granule proteins from the polymorphonuclear leucocyte. Biochem J. 1964 Mar;90(3):498–509. doi: 10.1042/bj0900498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B. P., Kummerow F. A., Nishida T. Acid phosphatases of rat polymorphonuclear leucocytes. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1045–1048. doi: 10.3181/00379727-122-31321. [DOI] [PubMed] [Google Scholar]
- ZUCKER-FRANKLIN D., HIRSCH J. G. ELECTRON MICROSCOPE STUDIES ON THE DEGRANULATION OF RABBIT PERITONEAL LEUKOCYTES DURING PHAGOCYTOSIS. J Exp Med. 1964 Oct 1;120:569–576. doi: 10.1084/jem.120.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]