Abstract
Background
Chronic lymphocytic leukemia (CLL) is a B-cell malignancy primarily diagnosed in older adults. For younger patients, treatment options often include regimens based on fludarabine, cyclophosphamide, and rituximab; however, at least 20% of patients exhibit resistance to these therapies. Ibrutinib, a covalent Bruton’s tyrosine kinase (BTK) inhibitor, has demonstrated enhanced safety compared to conventional treatments. This meta-analysis examines the efficacy and safety of ibrutinib in managing relapsed/refractory CLL.
Method
Relevant keywords were used to conduct a comprehensive search across online databases, including PubMed, Scopus, and Google Scholar. Data related to complete response (CR), overall response rate (ORR), and adverse events were extracted to evaluate the efficacy and safety of ibrutinib treatment. The results were presented in forest plots illustrating event rates and risk ratios with 95% confidence intervals (CI), while heterogeneity was assessed using I² statistics. Funnel plots were employed to examine potential publication bias visually.
Result
Twenty-one studies were included in this meta-analysis. Ibrutinib as a single-agent treatment was associated with a 9% complete response (CR) rate (95% CI: 5–14%) and a 77% overall response rate (ORR) (95% CI: 70–83%). When combined with other agents, ibrutinib achieved a CR rate of 21% (95% CI: 9–41%) and an ORR of 84% (95% CI: 80–88%). Adverse events were not significantly correlated with treatment outcomes. Funnel plots indicated no significant publication bias.
Conclusion
Single-agent ibrutinib has proven to be an effective therapy for patients with relapsed/refractory CLL. However, combining ibrutinib with other agents has demonstrated enhanced treatment efficacy. Further studies are needed to evaluate the safety profile of this therapeutic regimen thoroughly.
Keywords: Ibrutinib, Refractory CLL, Chronic lymphocyte leukemia, Relapsed CLL, Meta-analysis
Background
Chronic lymphocytic leukemia (CLL), a B-cell malignancy, continues to be an incurable disease. It is characterized by a proliferation of CD19+, CD5+, and CD23+ cells within the bone marrow, lymph nodes, spleen, and peripheral blood [1]. This disease predominantly affects the elderly, with a median age at diagnosis of 72 years. Between 2016 and 2020, the incidence rate of CLL was 4.6 per 100,000 individuals per year, with mortality rates highest among adults aged 75 and older, at 1.1 per 100,000 per year [2].
Despite advancements in therapeutic interventions over the past decade, CLL continues to lack a definitive cure. The current standard treatment approach for chronic CLL involves observation, though some studies suggest early intervention measures may be effective [3]. For younger CLL patients, chemoimmunotherapy, particularly with the FCR regimen (fludarabine, cyclophosphamide, rituximab), has traditionally served as the first-line treatment option [4]. Given that the median age at diagnosis for CLL patients is 72 years, many individuals are ineligible for chemoimmunotherapy due to advanced age and comorbidities. Additionally, at least 20% of CLL patients develop a chemo-refractory disease or resistance to targeted therapies, and approximately 10% progress to an aggressive lymphoma subtype known as Richter’s transformation [5].
Hopefully, recent advances in understanding the biological mechanisms underlying CLL have begun to inform clinical practice. Among these innovations are B-cell receptor (BCR) inhibitors, specifically Bruton tyrosine kinase (BTK) inhibitors, which offer promising therapeutic potential [4]. The BCR pathway is fundamental in regulating cellular processes vital for the survival and function of both normal and malignant B cells [6]. In CLL, dysregulated BCR signaling significantly drives disease progression, primarily by activating protein tyrosine kinases (PTKs) such as Lyn, Syk, and BTK. These PTKs display heightened activity and expression, facilitating malignant B cells’ unchecked proliferation and survival. This comprehension has driven progress in creating tailored inhibitors for these kinases. Among these, BTK is particularly noteworthy as a therapeutic target. Upon BCR stimulation, BTK activation initiates downstream signaling pathways that activate transcription factors critical for B-cell growth and differentiation [7–9].
Ibrutinib is a groundbreaking, oral, covalent, and irreversible inhibitor of BTK, designed to selectively bind to the cysteine residue CYS-481 at BTK’s active site, thereby effectively suppressing its enzymatic activity. Additionally, by blocking autophosphorylation at Tyr-223, ibrutinib disrupts downstream BCR signaling, ultimately inhibiting the proliferation and survival of malignant cells in CLL [7–9]. This targeted mechanism of action distinguishes ibrutinib as a valuable treatment option in the management of CLL. As the first once-daily, oral, covalent BTK inhibitor, ibrutinib has demonstrated enhanced efficacy and a more favorable safety profile compared to conventional chemoimmunotherapy regimens for both relapsed/refractory (R/R) and treatment-naïve CLL patients [10]. Despite its clinical advantages, the treatment is associated with certain adverse effects due to off-target kinase inhibition. This leads to discontinuation in approximately 16–24% of patients and dose adjustments in 13–23% of cases due to toxicity [11].
This systematic review and meta-analysis represents the first comprehensive evaluation aimed at rigorously assessing ibrutinib’s efficacy and safety profile in treating CLL. By synthesizing and analyzing data across multiple studies, we aim to provide a critical, evidence-based examination of ibrutinib’s therapeutic impact, including its benefits and potential risks. Significant heterogeneity in previous studies on ibrutinib, including variations in patient populations, study designs, and treatment outcomes, underscores the need for this meta-analysis to synthesize these diverse findings. This work offers a clearer understanding of ibrutinib’s role in CLL management, thereby supporting informed clinical decision-making and identifying areas where further research may be warranted.
Method
This systematic review and meta-analysis aimed to assess the real-world outcomes of ibrutinib in patients with relapsed or refractory CLL. Conducted in accordance with PRISMA guidelines, this study employed a rigorous protocol that included standardized checklists for comprehensive study searching and screening processes. The systematic review protocol was registered on the Open Science Framework (https://osf.io/dupky/) to enhance transparency and methodological integrity, ensuring adherence to high standards in research design and reporting.
Search strategy
We conducted a comprehensive search across multiple online databases, including PubMed, Scopus, and Google Scholar, utilizing relevant MeSH terms and keywords. Additionally, the references of pertinent articles were reviewed to ensure the inclusion of all eligible studies. The detailed search strategy is presented in Table 1.
Table 1.
Search strategy of current systematic review and meta-analysis
| Search engine | Search strategy | Results | Time |
|---|---|---|---|
| PubMed | ((ibrutinib[Title/Abstract]) OR (imbruvica[Title/Abstract]) OR (PCI-32765[Title/Abstract]) OR (BTK Inhibitor[Title/Abstract]) OR (Bruton’s Tyrosine Kinase Inhibitor[Title/Abstract]) OR (Bruton Tyrosine Kinase Inhibitor[Title/Abstract]) OR (ITK inhibitor[Title/Abstract]) OR (interleukin-2-inducible kinase inhibitor[Title/Abstract]) OR (interleukin-2 inducible kinase inhibitor[Title/Abstract])) AND ((cll[Title/Abstract]) OR (Chronic lymphocytic leukemia[Title/Abstract])) | 1679 | Aug 11th, 2023 |
| Scopus | ((TITLE-ABS-KEY (chronic AND lymphocytic AND leukemia) OR TITLE-ABS-KEY (cll))) AND ((TITLE-ABS-KEY (ibrutinib) OR TITLE-ABS-KEY (imbruvica) OR TITLE-ABS-KEY (pci 32765) OR TITLE-ABS-KEY (btk AND inhibitor) OR TITLE-ABS-KEY (bruton’s AND tyrosine AND kinase AND inhibitor) OR TITLE-ABS-KEY (bruton AND tyrosine AND kinase AND inhibitor) OR TITLE-ABS-KEY (itk AND inhibitor) OR TITLE-ABS-KEY (interleukin-2-inducible AND kinase AND inhibitor) OR TITLE-ABS-KEY (interleukin-2 AND inducible AND kinase AND inhibitor))) | 3221 | Aug 11th, 2023 |
Study selection
A comprehensive systematic search identified a total of 5328 studies. After the automatic removal of duplicates, 3909 unique studies remained. Two independent reviewers (R.K. and A.G.) screened the titles and abstracts to exclude irrelevant articles, resolving any disagreements through discussion. This initial screening excluded 3322 studies, leaving 587 studies for full-text assessment.
The full texts of the remaining studies were evaluated against the predefined inclusion criteria, which were: (1) publication in English, (2) a sample size of at least 20 participants, (3) the use of ibrutinib as monotherapy or in combination as a first-line treatment, (4) inclusion of refractory CLL patients, and (5) provision of sufficient data on at least one primary outcome, such as overall survival (OS), overall response rate (ORR) or complete response (CR). Studies not meeting these criteria were excluded.
Following a detailed review, 566 studies were excluded due to non-relevant outcomes, resulting in a final set of 21 studies with a cumulative sample size of 4,821 participants. These studies, published between 2014 and 2023, were included for in-depth analysis. The study selection process is illustrated in Fig. 1.
Fig. 1.
PRISMA diagram of the study selection process in this systematic review and meta-analysis. A comprehensive search across PubMed and Scopus databases, and Google Scholar, supplemented by citation tracking, yielded a total of 5,328 records, from which 1,419 duplicates were removed. Following title and abstract screening, 587 studies met the initial criteria for further review. Ultimately, 21 articles were included in the final analysis, with additional records excluded due to irrelevance to the study’s focus
Data extraction and quality assessment
Quality assessment was independently conducted by two reviewers (N.M. and M.B.) using the Cochrane tool for randomized controlled trials (RCTs) and the Joanna Briggs Institute (JBI) tool for cohort studies. Any disagreements were resolved through discussion. Two additional authors (H.K. and S.S.A.) performed data extraction from the included studies. Extracted data encompassed: (1) study details—region, year, and number of participants; (2) patient characteristics—age, sex, and group size; and (3) outcomes—counts for OS, ORR.
Statistical analysis
The primary outcomes evaluated in this study included OS, CR, and ORR. To further explore the safety profile, a meta-analysis was conducted to assess adverse events (AEs) across different doses of ibrutinib compared to control groups, along with a separate analysis specifically examining its use in combination therapy. A random-effects model was applied for studies with significant heterogeneity (I² > 50% or P < 0.1); otherwise, a fixed-effect model was used. Publication bias was evaluated using Begg’s funnel plot and Egger’s test, and sensitivity analysis was conducted to assess the robustness of the findings. All analyses were performed using Comprehensive Meta-Analysis software (CMA) software, version 3.0.
Result
Baseline characteristic
Among the 21 studies, a total of 4,821 participants were included. These studies comprised eight RCT [12–16], twelve cohort studies [17–27], and one case series [28]. The United States of America [16, 19, 23, 26–28], Japan [24], Italy [21], Sweden [25], France [20], Poland [18], China [15], Turkey [17], and multiple countries [12–14, 22, 29–32] were all locations where this research was carried out. The age of the patients varied from 21 to 90 years. All these studies investigated the effects of ibrutinib in patients with CLL, where participants were administered a standard daily dose of 420 milligrams of ibrutinib. The details of these studies are summarized in Table 2, which presents the characteristics of the included studies.
Table 2.
Baseline characteristics of included studies
| First author (year) | Study design | Participants | Country | Female (%) | Age Median (range) |
Type of the therapy | outcome | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Younes et al. (2019) [12] | RCT | 141 | Multinational | 38 | 65 (54–71) | Combination therapy | CR, ORR | Neutropenia, Anemia, Thrombocytopenia, Rash, HTN |
| Fraser et al. (2020) [13] | RCT | 578 | Multinational | Not specified | Not specified | Combination therapy | CR, ORR | Not specified |
| Hillmen et al. (2023) [14] | RCT | 652 | Multinational | 28.2 | Not specified | Monotherapy | CR, ORR | Neutropenia, Anemia, Thrombocytopenia, Diarrhea, HTN, Hemorrhage, Pneumonia, URTI |
| Huang et al. (2017) [15] | RCT | 160 | China | 29.4 | 66 (21–87) | Monotherapy | CR, ORR | Neutropenia, Anemia, Diarrhea, Thrombocytopenia, Asthenia/Fatigue, Rash, Nausea, URTI |
| Brown et al. (2015) [16] | RCT | 391 | U.S.A | Not specified | 67 | Combination therapy | CR, ORR | Neutropenia, Anemia, Thrombocytopenia, Asthenia/Fatigue, Diarrhea, Nausea, Pneumonia, URTI |
| Göçer et al. (2020) [17] | Cohort | 32 | Turkey | 36.4 | 65 (51–80) | Monotherapy | CR, ORR | Neutropenia, Anemia, Thrombocytopenia, Lymphocytosis, Diarrhea, Hemorrhage, Pneumonia |
| Pula et al. (2020) [18] | Cohort | 171 | Poland | 44.4 | 63 (39–85) | Monotherapy | CR, ORR | Neutropenia, Thrombocytopenia, AF, Diarrhea, Rash, HTN, Nausea, Hemorrhage, Pneumonia, URTI |
| Akhtar et al. (2017) [19] | Cohort | 144 | U.S.A | Not specified | Not specified | Monotherapy | CR, ORR | Not specified |
| Michallet et al. (2019) [20] | Cohort | 56 | France | 36 | 48 (35–64) | Monotherapy | CR, ORR | Not specified |
| Broccoli et al. (2021) [21] | Cohort | 46 | Italy | 32.6 | 62 (33–79) | Monotherapy | CR, ORR, OS | Not specified |
| Bonfiglio et al. (2023) [22] | Cohort | 98 | Multinational | 34.7 | 66 (33–86) | Monotherapy | CR, OS | Not specified |
| Byrd et al. (2015) [23] | Cohort | 132 | U.S.A | 22 | 64 (37–82) | Monotherapy | CR, ORR | Neutropenia, Thrombocytopenia, Lymphocytosis, Asthenia/Fatigue, AF, Diarrhea, HTN, Pneumonia |
| Omi et al. (2022) [24] | Cohort | 323 | Japan | 34.6 | 72 (33–92) | Monotherapy | ORR | Thrombocytopenia, Lymphocytosis, AF, Hemorrhage |
| Hansson et al. (2015) [25] | Cohort | 97 | Sweden | Not specified | 69 | Monotherapy | ORR | Neutropenia, AF, Diarrhea, Hemorrhage |
| Brown et al. (2015) [26] | Cohort | 33 | U.S.A | 16.7 | 62 (41–82) | Monotherapy | ORR | Not specified |
| Wierda et al. (2020) [27] | Cohort | 19 | U.S.A | Not specified | 60 (50–77) | Combination therapy | CR, ORR | Neutropenia, Anemia, AF, Diarrhea, Rash, HTN, Pneumonia |
| Burger et al. (2014) [28] | Case-series | 40 | U.S.A | 35 | 63.2 (35–82) | Combination therapy | CR | Neutropenia, Anemia, Asthenia/Fatigue, AF, Diarrhea, Nausea, URTI |
| Brown et al. (2023) [29] | RCT | 652 | Multinational | 31.5 | 67 (35–90) | Monotherapy | ORR | Neutropenia, Diarrhea, HTN, URTI, Covid-19 |
| Byrd et al. (2020) [30] | Cohort | 132 | Multinational | 25.8 | 66.5 (37–84) | Monotherapy | CR, OS |
Neutropenia HTN, Pneumonia |
| Byrd et al. (2021) [31] | RCT | 533 | Multinational | 26.8 | 66 (28–89) | Monotherapy | ORR | HTN, AF, Infection |
| Munir et al. (2019) [32] | RCT | 391 | Multinational | 32 | 67 (37–90) | Monotherapy | ORR |
Neutropenia HTN, Pneumonia, AF |
Abbreviations: CR complete response; ORR overall response rate; AF atrial fibrillation; HTN hypertension; RCT randomized controlled trial; URTI upper respiratory tract infection; OS overall survival
Monotherapy
OS
Pooled estimates of OS in relapsed/refractory CLL treated with ibrutinib monotherapy were calculated. It is shown that pooled OS was 0.56 (95% CI: 0.49–0.63), with a low heterogeneity (I2: 0%) (Fig. 2A). Moreover, Eggers’s test for asymmetry (p: 0.04) and the funnel plot showed significant evidence of publication bias (Fig. 2B).
Fig. 2.
A Forrest plot of pooled OS rate in patients treated with ibrutinib. B Funnel plot of pooled OS rate in patients treated with ibrutinib
CR
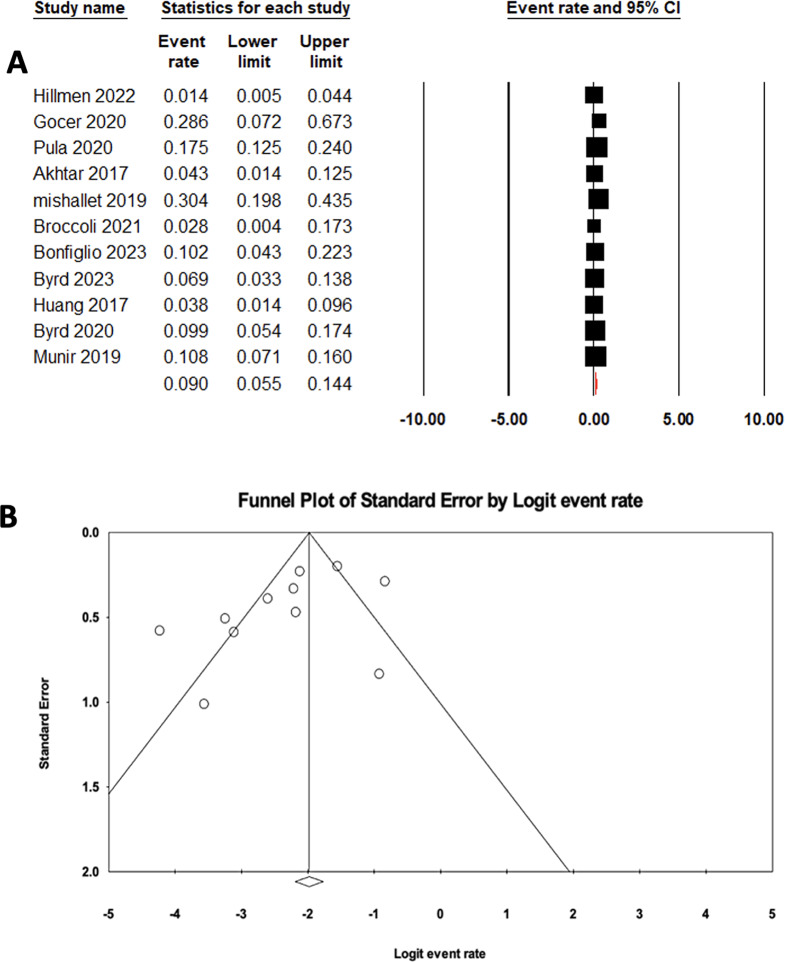
The pooled estimate for CR in relapsed/refractory CLL patients treated with ibrutinib monotherapy was 0.09 (95% CI: 0.05–0.14), with high heterogeneity (I2: 80.92%) (Fig. 3A). Moreover, Eggers’s test for asymmetry (p: 0.08) and the funnel plot showed no evidence of publication bias (Fig. 3B).
Fig. 3.
A Forrest plot of pooled CR rate in patients treated with ibrutinib. B Funnel plot of pooled CR rate in patients treated with ibrutinib
ORR
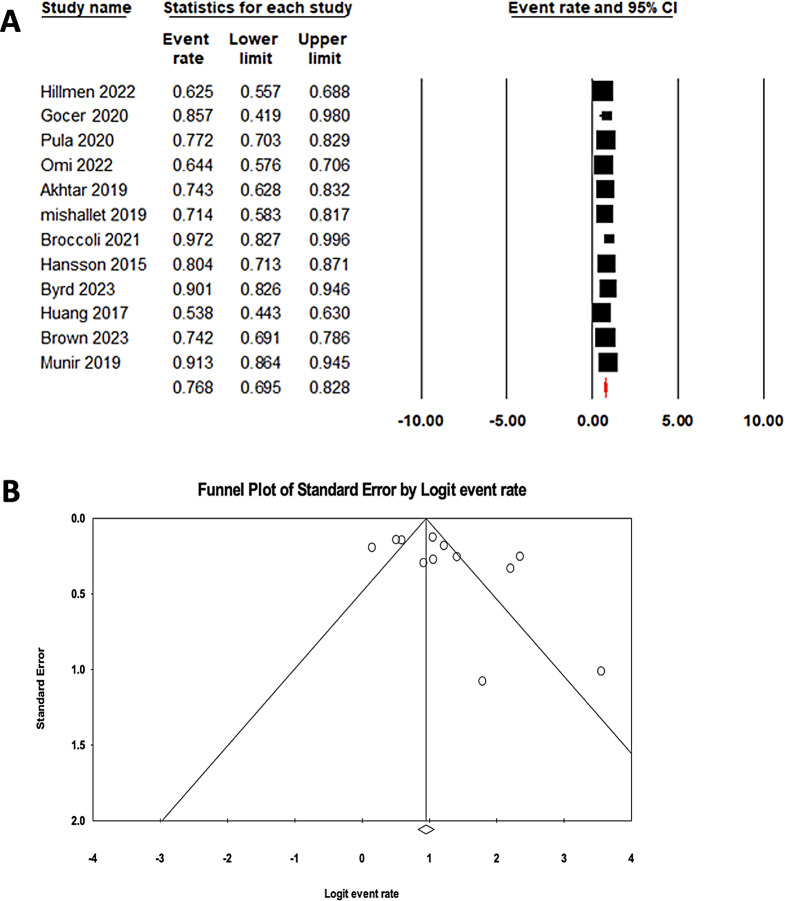
The pooled ORR for ibrutinib monotherapy in relapsed/refractory CLL patients was 0.77 (95% CI: 0.69–0.83), with substantial heterogeneity (I2: 87.83%) (Fig. 4A). Publication bias was not detected, as demonstrated by Egger’s test (p: 0.087) and the funnel plot (Fig. 4B).
Fig. 4.
A Forrest plot of pooled ORR in patients treated with ibrutinib. B Funnel plot of pooled ORR in patients treated with ibrutinib
Combination therapy
CR
In relapsed/refractory CLL patients receiving ibrutinib combination therapy, the pooled estimate of CR was 0.21 (95% CI: 0.09–0.41), showing high heterogeneity (I² = 84.00%) (Fig. 5A). Assessment for publication bias using Egger’s test (p = 0.098) and a funnel plot revealed no significant bias (Fig. 5B).
Fig. 5.
A Forrest plot of pooled CR rate in patients treated with ibrutinib combination therapy. B Funnel plot of pooled CR rate in patients treated with ibrutinib combination therapy
ORR
The pooled ORR for relapsed/refractory CLL patients treated with ibrutinib in combination therapy was 0.84 (95% CI: 0.80–0.88), also with high heterogeneity (I² = 83.18%) (Fig. 6A). Publication bias assessment using Egger’s test (p = 0.953) and a funnel plot indicated no bias (Fig. 6B).
Fig. 6.
A Forrest plot of pooled ORR in patients treated with ibrutinib combination therapy. B Funnel plot of pooled ORR in patients treated with ibrutinib combination therapy
Adverse events (AEs)
Grade 3 and 4 adverse events
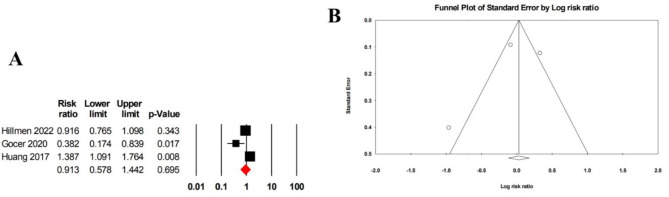
Three studies comprising 325 patients reported on grade 3 and 4 AEs, yielding a pooled risk ratio of 0.91 (95% CI: 0.58–1.44) with high heterogeneity (I² = 85.29) (Fig. 7A). Egger’s test for asymmetry (p = 0.693) and a funnel plot suggested no evidence of publication bias (Fig. 7B).
Fig. 7.
A Forrest plot of pooled risk ratio for grades 3 and in patients treated with ibrutinib monotherapy. B Funnel plot of pooled risk ratio for grades 3 and 4 in patients treated with ibrutinib monotherapy
Neutropenia
The pooled risk ratio for neutropenia, based on three studies with a total of 325 patients, was 0.92 (95% CI: 0.62–1.37), showing low heterogeneity (I² = 11.78) (Fig. 8A). Publication bias was not observed, as indicated by Egger’s test (p = 0.159) and the funnel plot (Fig. 8B).
Fig. 8.
A Forrest plot of pooled risk ratio for neutropenia in patients treated with ibrutinib monotherapy. B Funnel plot of pooled risk ratio for neutropenia in patients treated with ibrutinib monotherapy
Anemia
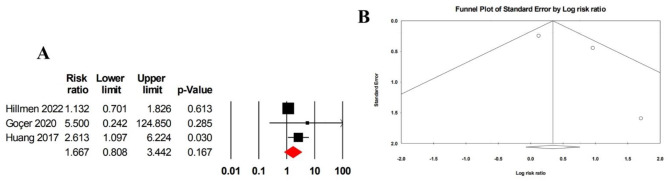
The pooled risk ratio for anemia, reported in three studies including 325 patients, was 1.67 (95% CI: 0.81–3.44), with moderate heterogeneity (I² = 42.61) (Fig. 9A). No evidence of publication bias was detected, based on Egger’s test (p = 0.406) and the funnel plot (Fig. 9B).
Fig. 9.
A Forrest plot of pooled risk ratio for anemia in patients treated with ibrutinib monotherapy. B Funnel plot of pooled risk ratio for anemia in patients treated with ibrutinib monotherapy
Thrombocytopenia
For thrombocytopenia, the pooled risk ratio was 1.50 (95% CI: 0.58–3.86), derived from three studies including 325 patients, with low heterogeneity (I² = 29.96) (Fig. 10A). Egger’s test (p = 0.975) and the funnel plot showed no indication of publication bias (Fig. 10B).
Fig. 10.
A Forrest plot of pooled risk ratio for thrombocytopenia in patients treated with ibrutinib monotherapy. B Funnel plot of pooled risk ratio for thrombocytopenia in patients treated with ibrutinib monotherapy
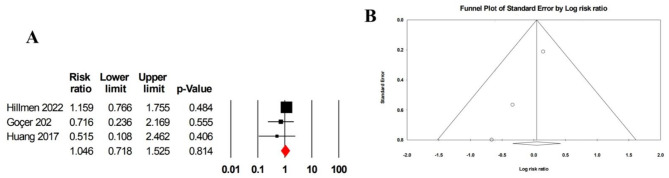
Diarrhea
The pooled risk ratio for diarrhea was 1.05 (95% CI: 0.72–1.52), based on three studies including 325 patients, with low heterogeneity (I² = 29.96) (Fig. 11A). However, Egger’s test (p = 0.005) and the funnel plot indicated significant evidence of publication bias (Fig. 11B).
Fig. 11.
A Forrest plot of pooled risk ratio for diarrhea in patients treated with ibrutinib monotherapy. B Funnel plot of pooled risk ratio for diarrhea in patients treated with ibrutinib monotherapy
Discussion
In this comprehensive systematic review and meta-analysis, we aimed to evaluate the impacts of ibrutinib on patients diagnosed with R/R CLL. This analysis included 4821 participants selected from 21 studies. The data analysis indicates that administering ibrutinib as a single-agent therapy to patients diagnosed with R/R CLL yielded noteworthy rates of OS, CR and ORR. Moreover, the co-administration of ibrutinib with other pharmacological treatments notably enhanced both the CR rate and ORR.
In our assessment, ibrutinib as monotherapy demonstrated satisfactory effectiveness in managing patients suffering from CLL. Our analysis found that in patients with R/R CLL treated with ibrutinib as a monotherapy, the rate of CR increased by 9% (95% CI: 5–14%). Additionally, the study noted a notable increase in the ORR among patients with R/R CLL who received ibrutinib as a single-agent therapy, reaching 77% (95% CI: 70–83%). A study conducted by Brown et al. [26] evaluated the efficacy of ibrutinib compared to ofatumumab in patients with previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Their demonstration showed that brutinib significantly improved Progression-free survival (PFS), OS, and ORR compared to ofatumumab in patients with CLL/SLL. As evaluated by the investigator, the highest ORR for ibrutinib against ofatumumab was 90% versus 25%. This included 8% of patients on ibrutinib attaining Partial response (PR) with lymphocytosis and 6% obtaining complete response. Also, Michallet et al. [20] undertook a study to assess the effectiveness of ibrutinib as a salvage therapy after allo-HSCT in 56 patients with CLL. Their investigation unveiled a documented ORR of 71% in people who were administered ibrutinib. Out of the individuals in this group, 41% showed a PR, while 30% achieved CR. Following the results of the previous studies, Göçer et al. [17] revealed that ibrutinib is a practical therapy choice for CLL and other B-cell lymphomas due to its favorable side effect profile and notable rates of complete or partial response. In their investigation involving 32 patients, of whom 11 were diagnosed with CLL, the researchers observed an ORR of 85.6%. This encompassed a CR rate of 28.5% and a PR rate of 57.1% during the final assessment of treatment with ibrutinib.
The findings of our study closely align with the results of three distinct studies carried out by Pula et al. [18], Byrd et al. [23], and Broccoli et al. [21] regarding the effectiveness of ibrutinib in treating CLL. Pula et al. [18] conducted observational research with a cohort of 171, in which they documented an ORR of 77.2%. Out of the total group, 30 individuals (17.5%) experienced a CR, while 62 patients (36.3%) experienced a PR to the treatment, demonstrating significant effectiveness. Byrd et al. [23] examined how treatment-naïve CLL patients responded to single-agent ibrutinib, finding that 84% had an ORR. Among the individuals in this group, 23% obtained CR, while 55% showed a PR, highlighting the significant effectiveness of the treatment in this particular demographic. Broccoli et al. [21] investigated the lasting efficacy of ibrutinib in patients with CLL, analyzing the results according to the order in which the drug was given. The ORR among patients who received ibrutinib as their initial treatment was 100%, consisting of one case of CR and nine cases of PR. In patients receiving ibrutinib as a second or later line of therapy, the ORR was 97.2%, comprising one case of CR and 34 cases of PR, highlighting consistent and favorable responses across different stages of disease progression.
Additionally, our analysis revealed OS rate of 56.4% (95% CI: 49.2–63.4%), highlighting the consistent efficacy of ibrutinib monotherapy in improving survival outcomes among patients with CLL across various treatment settings. This finding is further supported by previous studies. Broccoli et al. [21] reported that the median OS was not reached for patients treated with ibrutinib as a frontline therapy, while those in R/R settings achieved a median OS of 4.9 years, emphasizing its effectiveness even in advanced disease stages. Bonfiglio et al. [22] reinforced the survival advantages associated with long-term ibrutinib use in a real-world setting. Similarly, Byrd et al. [30] documented a 7-year OS of 84% in the frontline setting and 55% in R/R cases, demonstrating the durable benefits of ibrutinib monotherapy across various treatment contexts. Collectively, these findings affirm the consistent and significant survival benefits provided by ibrutinib monotherapy in the management of CLL.
However, specific investigations yield results that differ from our observed outcomes in assessing the effectiveness of ibrutinib for CLL. Hillmen et al. [14] compared zanubrutinib with ibrutinib in R/R CLL/SLL patients. They found that zanubrutinib had a higher ORR (78%; 95% CI: 72–83%) than ibrutinib (62%; 95% CI: 55–69%).
Following our analysis, we found that co-administering ibrutinib alongside other pharmacological treatments increases the CR rate by 21% (95% CI: 9–41%). Moreover, the study highlighted a significant rise in the ORR among patients with R/R CLL who were treated with ibrutinib in combination with other medications. Co-administration of ibrutinib alongside other medications resulted in an 84% increase in the ORR (95% CI: 80–88%). Wierda et al. [27] conducted a Phase II clinical study to evaluate the effectiveness of a combination therapy incorporating ibrutinib and venetoclax as the initial treatment for CLL, and they demonstrated statistically significant outcomes. They found that 97% of the 164 enrolled patients had a significant ORR (95% CI: 93–99%), and 76 patients achieved CR, accounting for 46% (95% CI: 39–54%) [27]. In a five-year longitudinal study, Fraser et al. [13] investigated the efficacy of a combined regimen involving ibrutinib and bendamustine plus rituximab among patients diagnosed with R/R CLL/SLL. The findings revealed a notable escalation in the CR rate, achieving 40.8%. Moreover, the cohort treated with ibrutinib alongside rituximab exhibited a marked enhancement in the ORR at 87.2%, significantly surpassing the 66.1% seen in the placebo plus rituximab group (p < 0.0001). Despite further inquiries investigating the concurrent use of ibrutinib alongside rituximab [28] yielding notable findings, the combination of ibrutinib with nivolumab [12] in patients with relapsed CLL did not demonstrate a statistically significant CR rate comparable to previous studies.
Ibrutinib’s side effects were evaluated in three studies, which included 325 individuals. The events in Grades 3 and 4 exhibited no significant differences but displayed substantial variation, necessitating additional examination. Neutropenia, anemia, and thrombocytopenia showed consistent patterns, suggesting a uniform effect. Anemia tended to increase risk, while the difference was not statistically significant. The risk of diarrhea, despite minor variation, was affected by publication bias. These findings emphasize the importance of conducting thorough assessments, particularly of severe events and nuanced observations on anemia, while considering the potential bias in published data on diarrhea. Further comprehensive investigations are essential for gaining a more profound understanding.
This systematic review and meta-analysis employed a comprehensive search across multiple databases, minimizing selection bias and ensuring the inclusion of relevant studies. Despite filling a significant gap in the literature by evaluating the impact of ibrutinib on CR and ORR in patients with R/R CLL, the study has certain limitations. Notably, the substantial heterogeneity among the included studies constrained the analyses. Variations in treatment regimens, with each study utilizing distinct combination therapies, posed challenges for direct comparisons and limited the feasibility of detailed subgroup analyses. Furthermore, incomplete reporting of survival outcomes in some studies restricted the assessment of survival data, with only PFS and OS metrics available in several cases. Future research is recommended to validate these findings and strengthen the results by achieving greater statistical robustness.
Conclusion
Single-agent ibrutinib showed significant efficacy with a 9% complete response rate and an impressive 77% overall response rate for R/R CLL patients. The co-administration of ibrutinib with other therapies led to a significant 21% rise in the rate of complete response and a remarkable 84% increase in the overall response rate. Evaluating ibrutinib’s adverse effects revealed varying trends in Grade 3 and 4 events while highlighting consistent neutropenia, anemia, and thrombocytopenia patterns. Nevertheless, the differences in results and the heterogeneity in comparisons with other medications or combinations emphasize the need for additional research to improve treatment methods for patients with R/R CLL, focusing on both effectiveness and adverse effects.
Acknowledgments
We appreciate all authors of the included studies.
Author contributions
Conception and study design: N.D, MA.K; Protocol: H.N; Systematic search: MA.K, study selection: MA.K, R.K, A.G; Data extraction: MA.K, H.K, SS.A; Quality assessment: N.M M.B; Data analysis: M.N, S.H; Drafting the manuscript: MA.K, H.N, R.K, A.G, H.K, SS.A, N.M, M.B; critical revision: MA.K, N.D, M.N.S.H, S.M all authors approved the submitted version of the manuscript.
Funding
None.
Data availability
Data is available upon request from corresponding author
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohammad Amin Karimi, Hanieh Norooziseyedhosseini and Reza Khademi contributed to this work equally.
Contributor Information
Mahdyieh Naziri, Email: nazirimahdyieh@yahoo.com.
Niloofar Deravi, Email: niloofarderavi@sbmu.ac.ir.
References
- 1.Alsagaby SA, et al. In silico investigations identified Butyl Xanalterate to competently target CK2α (CSNK2A1) for therapy of chronic lymphocytic leukemia. Sci Rep. 2022;12:17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braish J, Cerchione C, Ferrajoli A. An overview of prognostic markers in patients with CLL. Front Oncol. 2024;14:1371057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langerbeins P, et al. The CLL12 trial: ibrutinib vs placebo in treatment-naïve, early-stage chronic lymphocytic leukemia. Blood. 2022;139:177–87. [DOI] [PubMed] [Google Scholar]
- 4.Jain N, O’Brien S. Targeted therapies for CLL: practical issues with the changing treatment paradigm. Blood Rev. 2016;30(3):233–44. [DOI] [PubMed] [Google Scholar]
- 5.Iyer P, Wang L. Emerging therapies in CLL in the era of precision medicine. Cancers (Basel). 2023;15(5):1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavez JC, Sahakian E, Pinilla-Ibarz J. Ibrutinib: an evidence-based review of its potential in the treatment of advanced chronic lymphocytic leukemia. Core Evid. 2013;8:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akinleye A, et al. Ibrutinib and novel BTK inhibitors in clinical development. J Hematol Oncol. 2013;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman SE, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt V, et al. The promising impact of ibrutinib, a Bruton’s tyrosine kinase inhibitor, for the management of lymphoid malignancies. Pharmacotherapy. 2014;34:303–14. [DOI] [PubMed] [Google Scholar]
- 10.Abrisqueta P, et al. Real-world characteristics and outcome of patients treated with single-agent ibrutinib for chronic lymphocytic leukemia in Spain (IBRORS-LLC Study). Clin Lymphoma Myeloma Leuk. 2021;21:e985–e999. [DOI] [PubMed] [Google Scholar]
- 11.Frustaci AM, et al. Next generation BTK inhibitors in CLL: evolving challenges and new opportunities. Cancers (Basel). 2023;15:1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Younes A, et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol. 2019;6:e67–e78. [DOI] [PubMed] [Google Scholar]
- 13.Fraser GA, et al. Final 5-year findings from the phase 3 HELIOS study of ibrutinib plus bendamustine and rituximab in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Leuk Lymphoma. 2020;61:3188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillmen P, et al. Zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma: interim analysis of a randomized phase III trial. J Clin Oncol. 2023;41:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, et al. Ibrutinib versus rituximab in relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma: a randomized, open-label phase 3 study. Cancer Med 2018;7:1043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JR, et al. Updated efficacy including genetic and clinical subgroup analysis and overall safety in the phase 3 RESONATETM trial of ibrutinib versus ofatumumab in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood. 2014;124:3331. [Google Scholar]
- 17.Göçer M, Kurtoğlu E. Safety and efficacy analysis of ibrutinib in 32 patients with CLL and various B-cell lymphomas: real-world data from a single-center study in Turkey. Blood Res. 2020;55(4):206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pula B, et al. Long-term efficacy of ibrutinib in relapsed or refractory chronic lymphocytic leukemia: results of the polish adult leukemia study group observational study. Anticancer Res 2020;40:4059–66. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar OS, et al. Disease progression on ibrutinib therapy is associated with a poor clinical outcome in chronic lymphocytic leukemia (CLL) patients managed in standard clinical practice. Blood. 2017;130:5350. [Google Scholar]
- 20.Michallet M, et al. Ibrutinib as a salvage therapy after allogeneic HCT for chronic lymphocytic leukemia. Bone Marrow Transplant. 2020;55:884–90. [DOI] [PubMed] [Google Scholar]
- 21.Broccoli A, et al. Long-term efficacy and safety of ibrutinib in the treatment of CLL patients: a real life experience. J Clin Med. 2021;10:5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonfiglio S, et al. BTK and PLCG2 remain unmutated in one third of patients with CLL relapsing on ibrutinib. Blood Adv. 2023;7:2794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrd JC, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood J Am Soc Hematol. 2015;125:2497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omi A, et al. Efficacy and safety of ibrutinib in relapsed/refractory CLL and SLL in Japan: a post-marketing surveillance. J Clin Exp Hematop. 2022;62:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansson L, et al. Real-world results on ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia (CLL): data from 97 Swedish patients treated in a compassionate use program. Blood. 2015;126:1745. [Google Scholar]
- 26.Brown JR, et al. The Bruton tyrosine kinase inhibitor ibrutinib with chemoimmunotherapy in patients with chronic lymphocytic leukemia. Blood J Am Soc Hematol. 2015;125:2915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wierda WG, et al. Transcend CLL 004: phase 1 cohort of lisocabtagene maraleucel (liso-cel) in combination with ibrutinib for patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Blood. 2020;136:39–40. [Google Scholar]
- 28.Burger JA, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown, J.R., et al., Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2023;388(4):319–32. 10.1056/NEJMoa2211582 [DOI] [PubMed]
- 30.Byrd, J.C., et al., Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase Ib/II PCYC-1102 study. Clin Cancer Res. 2020;26(15):3918–27. 10.1158/1078-0432.ccr-19-2856 [DOI] [PMC free article] [PubMed]
- 31.Byrd, J.C., et al., Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441–52. 10.1200/jco.21.01210 [DOI] [PMC free article] [PubMed]
- 32.Munir, T., et al., Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–63. 10.1002/ajh.25638 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request from corresponding author