Abstract
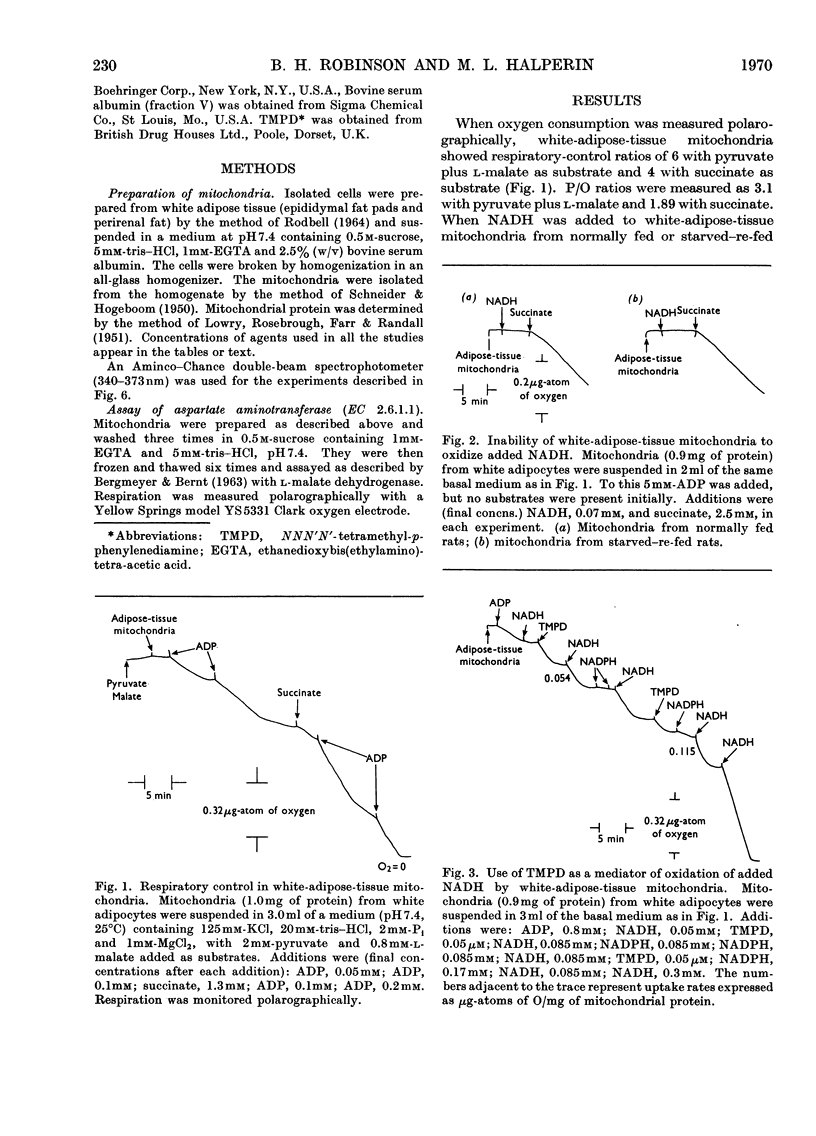
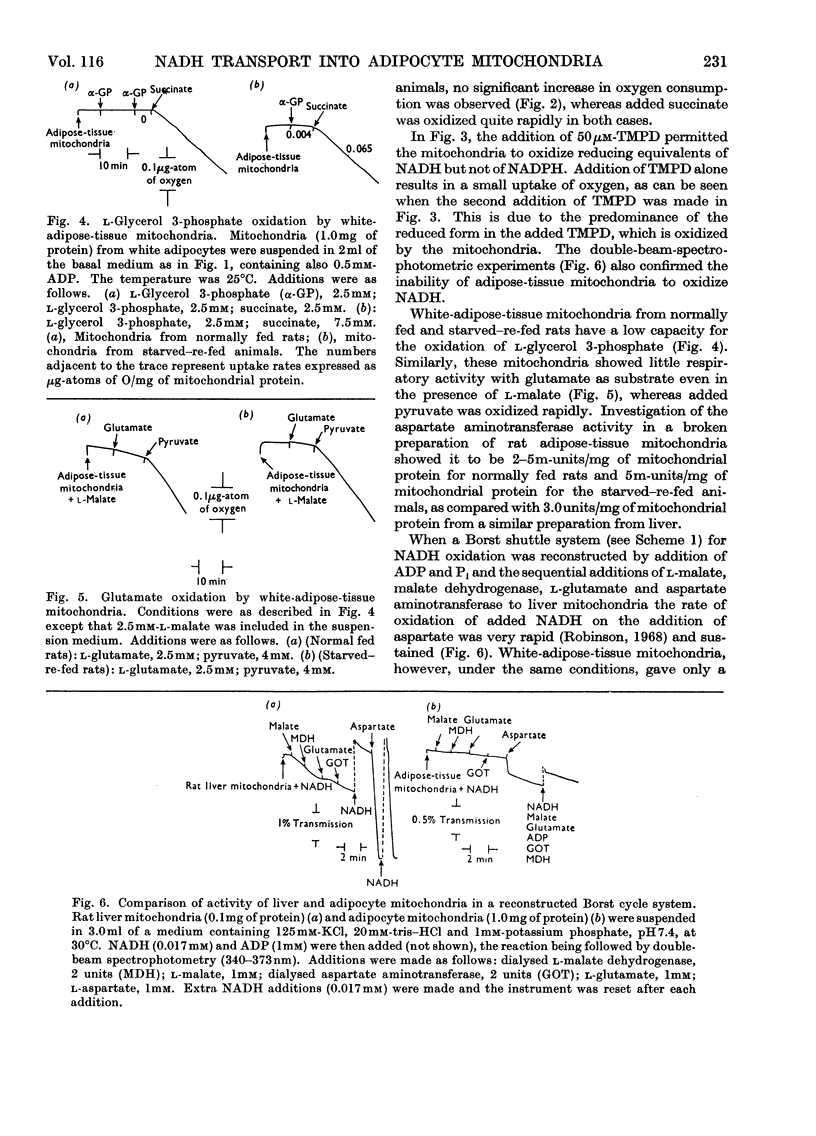
Mitochondria from rat white adipose tissue were prepared, exhibiting good respiratory control and P/O ratios. They would not oxidize NADH unless NNN′N′-tetramethyl-p-phenylenediamine was added as a carrier of reducing equivalents. These mitochondria were found to oxidize neither l-glycerol 3-phosphate nor l-glutamate plus l-malate at significant rates. The activity of aspartate aminotransferase in these mitochondria was found to be low compared with that found in rat liver mitochondria. As a consequence of this, the adipose-tissue mitochondria exhibited very low rates of cytoplasmic NADH oxidation in a reconstituted Borst (1962) cycle compared with liver mitochondria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORST P. The aerobic oxidation of reduced diphosphopyridine nucleotide formed by glycolysis in Ehrlich ascites-tumour cells. Biochim Biophys Acta. 1962 Feb 26;57:270–282. doi: 10.1016/0006-3002(62)91120-4. [DOI] [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- Flatt J. P., Ball E. G. Studies on the metabolism of adipose tissue. XIX. An evaluation of the major pathways of glucose catabolism as influenced by acetate in the presence of insulin. J Biol Chem. 1966 Jun 25;241(12):2862–2869. [PubMed] [Google Scholar]
- HENSON C. P., CLELAND W. W. KINETIC STUDIES OF GLUTAMIC OXALOACETIC TRANSAMINASE ISOZYMES. Biochemistry. 1964 Mar;3:338–345. doi: 10.1021/bi00891a007. [DOI] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., REIM M. Steady state equilibria of some DPN-linked reactions and the oxidation/reduction state of the DPN/DPNH system in the cytoplasmatic compartment of liver cells in vivo. Biochem Biophys Res Commun. 1961 Mar 10;4:159–162. doi: 10.1016/0006-291x(61)90262-5. [DOI] [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- LEHNINGER A. L. Phosphorylation coupled to oxidation of dihydrodiphosphopyridine nucleotide. J Biol Chem. 1951 May;190(1):345–359. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PURVIS J. L., LOWENSTEIN J. M. The relation between intra- and extramitochondrial pyridine nucleotides. J Biol Chem. 1961 Oct;236:2794–2803. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]


