Abstract
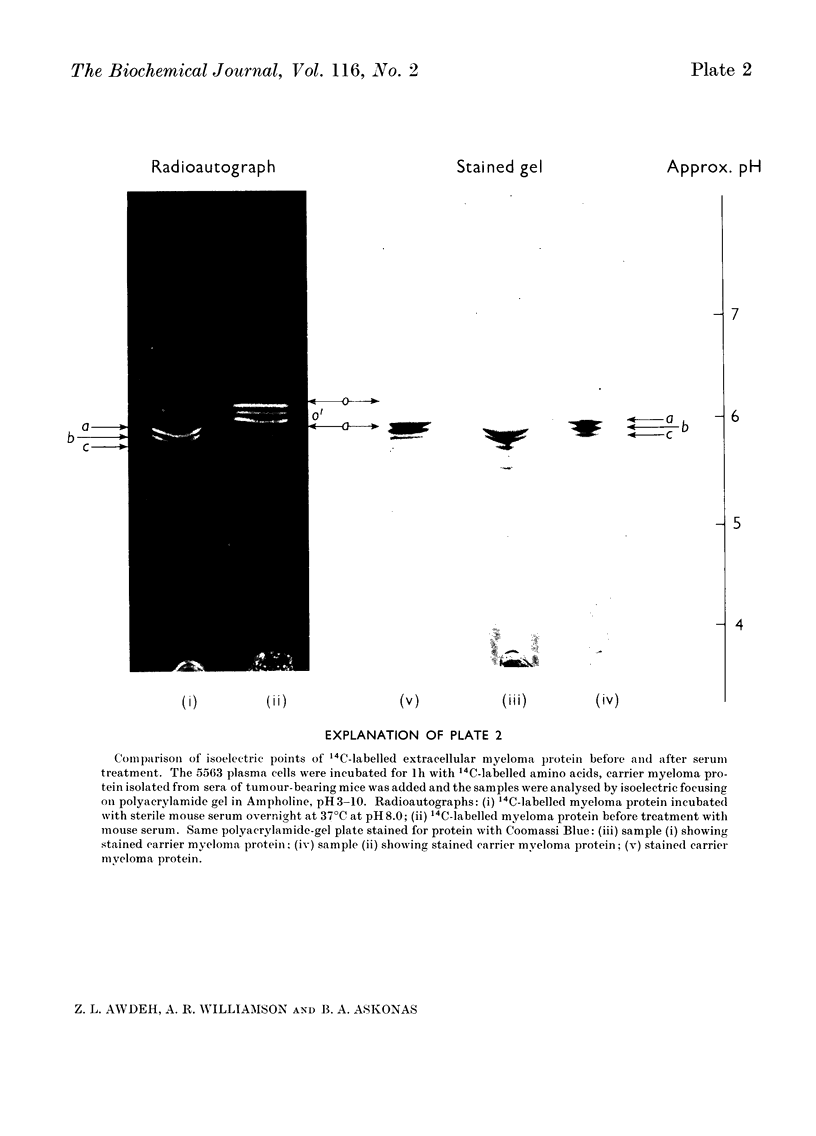
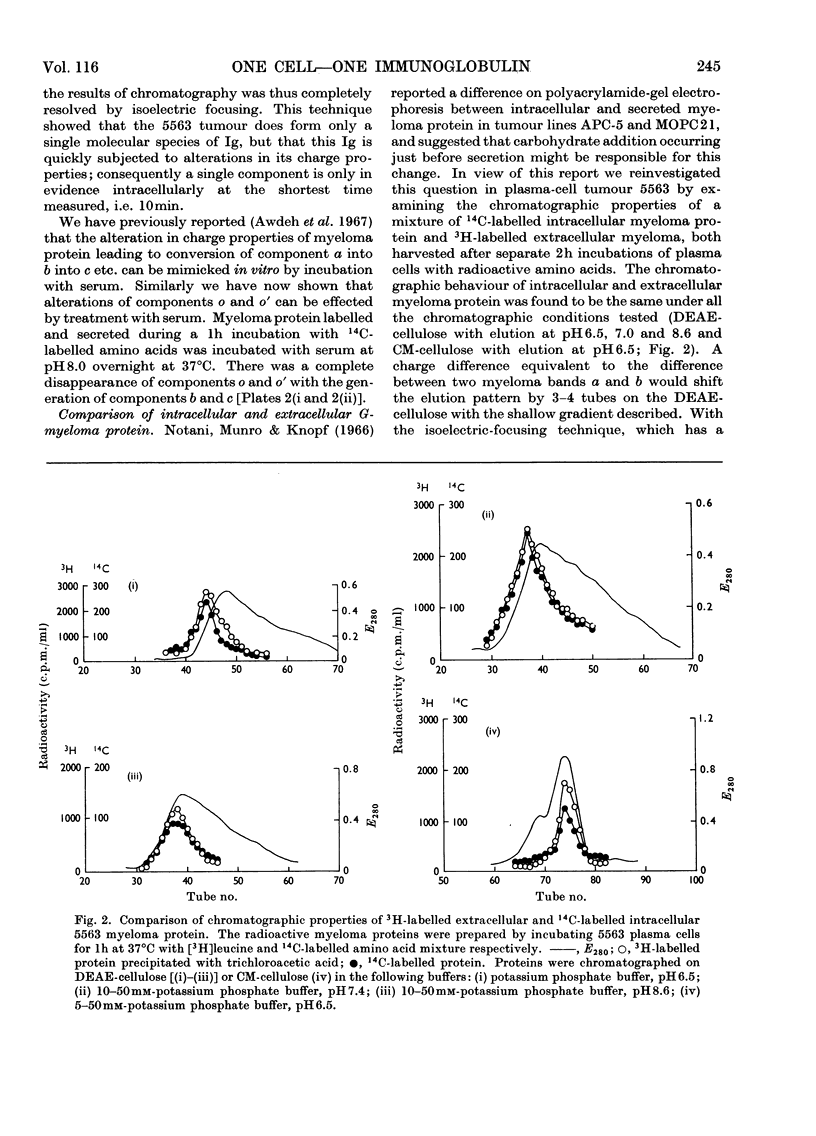
Plasma-cell tumour 5563 forms a single molecular species of immunoglobulin IgG2a, i.e. one variant of heavy chain and one variant of light chain. The molecules formed are labile and undergo alterations in charge properties, which rapidly lead to heterogeneity of the myeloma protein after synthesis. The single immunoglobulin species originally formed is found only after the shortest time-intervals tested, i.e. 10min incubation. Two types of changes in charge properties take place: (1) The originally formed protein (component o) is converted via an intermediate o′ into the most basic form of 5563 myeloma protein found in serum (component a). Charge differences between these components are suppressed at pH8.9, but can be studied by chromatography at pH6.5 or by analysis of isoelectric points by isoelectric focusing in polyacrylamide gel. The conversion of components o and o′ into component a apparently commences soon after assembly of the molecules and proceeds to completion extracellularly. (2) The second type of charge difference that distinguishes components a, b, c and d is exhibited over the pH range 6.0–8.9, but not at acid pH, and has been studied by electrophoresis at pH8.9, by chromatography and by isoelectric focusing. Conversion of component a into components b, c, d and e is only partial so that all five components can be found at decreasing concentrations in serum. Both types of charge alteration can be effected in vitro in the presence of serum, with optimum pH8.0. None of the charge differences could be attributed to the secretion process, since a component with the same isoelectric point as component o was found in secreted myeloma protein (1h). We have found no evidence to support the idea that the first type of change from component o to component a is due to ring formation of N-terminal [14C]glutamine into pyrrolid-2-one-5-carboxylic acid; however, our findings do not exclude this process happening very rapidly to a precursor of component o, possibly the polypeptide chain during or immediately after synthesis. In studying this point we noted that not only the heavy chains but also the κ-type light chain of mouse 5563 myeloma protein have a blocked N-terminus.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A. A study on globulin formation by plasma-cell neoplasm (5563) transplantable in mice. Biochem J. 1961 Apr;79:33–43. doi: 10.1042/bj0790033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awdeh Z. L., Askonas B. A., Williamson A. R. The homogeneous-gamma-G-immunoglobulin produced by mouse plasmacytoma 5563 and its subsequent heterogeneity in serum. Biochem J. 1967 Feb;102(2):548–553. doi: 10.1042/bj1020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awdeh Z. L., Williamson A. R., Askonas B. A. Heavy and light chains of a homogeneous immunoglobulin-G. FEBS Lett. 1969 Nov 29;5(4):275–278. doi: 10.1016/0014-5793(69)80367-4. [DOI] [PubMed] [Google Scholar]
- Awdeh Z. L., Williamson A. R., Askonas B. A. Isoelectric focusing in polyacrylamide gel and its application to immunoglobulins. Nature. 1968 Jul 6;219(5149):66–67. doi: 10.1038/219066a0. [DOI] [PubMed] [Google Scholar]
- Bernfield M. R., Nestor L. The enzymatic conversion of glutaminyl-tRNA to pyrrolidone carboxylate-tRNA. Biochem Biophys Res Commun. 1968 Dec 9;33(5):843–849. doi: 10.1016/0006-291x(68)90238-6. [DOI] [PubMed] [Google Scholar]
- Cebra J. J., Colberg J. E., Dray S. Rabbit lymphoid cells differentiated with respect to alpha-, gamma-, and mu- heavy polypeptide chains and to allotypic markers Aa1 and Aa2. J Exp Med. 1966 Mar 1;123(3):547–558. doi: 10.1084/jem.123.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- Feinstein A. Use of charged thiol reagents in interpreting the electrophoretic patterns of immune globulin chains and fragments. Nature. 1966 Apr 9;210(5032):135–137. doi: 10.1038/210135a0. [DOI] [PubMed] [Google Scholar]
- LEVY A. L. A paper chromatographic method for the quantitative estimation of amino-acids. Nature. 1954 Jul 17;174(4420):126–127. doi: 10.1038/174126a0. [DOI] [PubMed] [Google Scholar]
- Moav B., Harris T. N. Pyrrolid-2-one-5 carboxylic acid involvement in the biosynthesis of rabbit immunoglobulin. Biochem Biophys Res Commun. 1967 Dec 15;29(5):773–776. doi: 10.1016/0006-291x(67)90285-9. [DOI] [PubMed] [Google Scholar]
- Notani G. W., Munro A. J., Knopf P. M. A charge difference between an intracellular and secreted mouse myeloma globulin. Biochem Biophys Res Commun. 1966 Nov 22;25(4):395–401. doi: 10.1016/0006-291x(66)90218-x. [DOI] [PubMed] [Google Scholar]
- Perham R., Appella E., Potter M. Light chains of mouse myeloma proteins: partial amino acid sequence. Science. 1966 Oct 21;154(3747):391–393. doi: 10.1126/science.154.3747.391. [DOI] [PubMed] [Google Scholar]
- Pernis B., Chiappino G., Kelus A. S., Gell P. G. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press E. M., Piggot P. J., Porter R. R. The N- and c-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG. Biochem J. 1966 May;99(2):356–366. doi: 10.1042/bj0990356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Bockman R. S. Studies on the proteolytic activity of gamma-globulin preparations. Biochem J. 1967 Feb;102(2):554–563. doi: 10.1042/bj1020554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüde E., Givol D. A blocked N-terminal residue in the light chain of rabbit immunoglobulin G. Biochem J. 1968 Apr;107(4):449–453. doi: 10.1042/bj1070449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A. R., Askonas B. A. Biosynthesis of immunoglobulins: the separate classes of polyribosomes synthesizing heavy and light chains. J Mol Biol. 1967 Jan 28;23(2):201–216. doi: 10.1016/s0022-2836(67)80027-5. [DOI] [PubMed] [Google Scholar]





