Abstract
Objective
Both early and late age at menarche have been associated with various health issues and may influence the risk of infertility. This present study investigated the relationship between age at menarche and infertility risk.
Methods
This study follows PRISMA guidelines. Databases including PubMed, Scopus, Web of Science, Embase, and Cochrane were searched in December 2024. Odds ratios with 95% confidence intervals were estimated using a random-effects model. Heterogeneity was assessed with the I2 index and chi-square, and publication bias was evaluated using Egger’s test and a funnel plot. Sensitivity analysis and meta-regression examined study impact and variable influence on heterogeneity.
Results
Out of 7,267 articles screened, 18 primary studies were included, yielding 21 pieces of evidence. The odds ratio (OR) for infertility in the late menarche group compared to the normal menarche group was 1.44 (95% CI: 0.98–2.10), while the OR for the early menarche group versus the normal menarche group was 0.98 (95% CI: 0.68–1.42). Additionally, the OR for infertility in the early menarche group compared to the late menarche group was 0.77 (95% CI: 0.55–1.06). For primary infertility, the OR for the late menarche group relative to the normal menarche group was 1.98 (95% CI: 1.02–3.85), whereas the OR for the early menarche group compared to the late menarche group was 0.59 (95% CI: 0.36–0.97).
Conclusion
Although the overall meta-analysis lacked statistical significance, subgroup analysis revealed a notable association between late menarche and primary infertility. Women with late menarche had higher odds of infertility, supporting a dose-responsive relationship. The observed 44% increase in infertility odds highlights late menarche as a potential risk factor, warranting further investigation into its implications for reproductive health.
Keywords: Age at menarche, Menstruation, Infertility, Fertility, Meta-analysis, Involuntary childlessness
Introduction
Infertility is defined as the inability to achieve pregnancy after 12 months of unprotected intercourse, which is associated with psychological distress, physical stress, and medical detriments in couples [1–4]. Globally, an estimated 9% of women experience infertility, with secondary infertility being more prevalent [4–6]. Infertility rates are higher in Eastern Europe, North Africa, and the Middle East [2, 5], reflecting geographical differences, which are attributed to various environmental, cultural, social, and economic factors, as well as access to healthcare systems [6, 7]. While various factors contribute to infertility—including age, body mass index (BMI), smoking, and stress—reproductive development, particularly the timing of menarche, has emerged as a potential determinant [1, 4, 8, 9].
Menarche, the onset of menstruation, marks the beginning of ovulation and fertility but does not guarantee them [10–12]. Menarche between 12 and 14 years of age is now considered clinically normal [13], though some researchers have defined other thresholds [14, 15]. The average age at menarche has declined by five years since the mid-19th century in most developed countries and continues to decrease globally [16–19]. Early and late menarche have been linked to multiple adverse health outcomes [7]. Early menarche is associated with increased risks of breast cancer [10, 20], cardiovascular related mortality and diseases [21], and mental health disorders [22, 23], while late menarche has been linked to osteoporosis and bone fractures [14, 24].
Despite increasing evidence on the impact of menarche timing on fertility and pregnancy outcomes, its association with infertility remains unclear [10, 25, 26]. While some studies suggest that both early and late menarche may be linked to infertility, findings have been inconsistent across populations and study designs [4, 10, 26, 27]. Given the conflicting evidence, we conducted this systematic review and meta-analysis to provide a comprehensive evaluation of the relationship between age at menarche and infertility. To our knowledge, this is the first systematic review and meta-analysis to specifically investigate this association.
Materials and methods
This study was designed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [28].
Inclusion and exclusion criteria
The inclusion and exclusion criteria were structured using PICO. The review adhered to pre-defined objectives and eligibility criteria to ensure consistency and focus. We included every peer-reviewed observational case-control, cohort, and cross-sectional study that reported age at menarche in fertile and infertile women, categorized into groups of early and late menarche or early, normal, and late menarche. Only studies in English and Persian from inception until December 6, 2024, were included. Case reports, case series, theses, and conference abstracts were excluded.
Infertility was defined as the failure to achieve a pregnancy after 12 months or more of regular unprotected sexual intercourse. The age at menarche varies across populations due to numerous influencing factors and may also differ within the same population over time. To address this variability, we included only studies that explicitly classified their samples into categories such as early, normal, and late menarche. This approach ensured that each study compared its population internally and reported its findings on the relationship between early, normal, and late menarche and infertility.
Search strategy
Databases including PubMed, Scopus, Embase, Web of Science, Cochrane, and the search engine Google Scholar were searched using specified MeSH and non-MeSH terms, along with the operators OR and AND. The Persian database Scientific Information Database (SID) was searched using Persian keywords for “Age at menarche”, “Infertility”, and “Fertility”. We also carefully reviewed the references of included studies to enhance the sensitivity of our search process. No restrictions were applied to the search. For studies with incomplete information, the authors were contacted to obtain full details. EndNote X9 (Thomson Reuters, America) was used to manage the studies. An example of the PubMed search strategy is as follows:
((“menarch“[All Fields] OR “menarchal“[All Fields] OR “menarche“[MeSH Terms] OR “menarche“[All Fields] OR “menarcheal“[All Fields] OR “menarches“[All Fields]) AND (“infertiles“[All Fields] OR “infertilities“[All Fields] OR “infertility“[MeSH Terms] OR “infertility“[All Fields] OR “infertile“[All Fields] OR “infertility s“[All Fields])) OR ((“menarch“[All Fields] OR “menarchal“[All Fields] OR “menarche“[MeSH Terms] OR “menarche“[All Fields] OR “menarcheal“[All Fields] OR “menarches“[All Fields]) AND (“fertiles“[All Fields] OR “fertility“[MeSH Terms] OR “fertility“[All Fields] OR “fertile“[All Fields] OR “fertilities“[All Fields]))
Study selection
We initially removed duplicates using EndNote software, then screened the studies based on title and abstract to eliminate irrelevant records. The full text of the selected studies was carefully evaluated to review the predefined inclusion and exclusion criteria. This process was conducted independently by two authors, and in case of disagreement, the study was discussed with a third author who made the final decision.
Quality assessment
The risk of bias was assessed using the Newcastle-Ottawa Scale (NOS) checklist [29]. Assessment was done independently by two authors. This checklist includes three sections, namely Selection, Comparability and Exposure, and globally score studies from 0 to 9 points. Studies that obtained a score < 5 points were excluded. Selection criteria maximum score is 4, Comparability is 2 and Exposure is 3.
Data extraction
Data extraction was independently performed by two authors using a pre-defined form. In case of disagreement, the study was discussed with a third author who made the final decision. The extracted data from each study included the following: the first author’s name, study title, year of publication, country of study, study type, type of infertility (primary or secondary or both), number of participants in the fertile and infertile groups, mean age of the fertile and infertile groups with standard deviation, definition of early, normal, and late menarche, and the number of fertile and infertile samples in the groups with early, normal, and late menarche.
Statistical analysis
Data analysis was performed using Stata Ver. 11 software. To estimate the odds ratio (OR) using a two-by-two table, the number of individuals with early, normal, and late menarche was extracted separately for the infertile and fertile women groups from each primary study. Using a random effects model and inverse variance, the ORs were estimated for early menarche compared to normal menarche, late menarche compared to normal menarche, and early menarche compared to late menarche, with a 95% confidence interval (CI). The significance criterion for the OR between the two groups (with and without infertility) was the exclusion of the number one from the upper and lower confidence interval of the OR. Heterogeneity between the results of primary studies was assessed using the I-square index, chi-square, and publication bias with the Egger test and funnel plot. Sensitivity analysis was also conducted to examine the impact of each primary study on the overall estimate. Meta-regression was performed to investigate the impact of the criteria for early menarche, late menarche, and type of infertility (primary/secondary) on heterogeneity among the primary studies.
Results
Based on the search strategy across the mentioned databases, 7276 articles were retrieved. Using EndNote software, 3148 duplicate articles were removed. Subsequently, 3881 articles were excluded after screening the titles and abstracts. Full-text evaluation was performed for 247 articles, and a total of 229 articles were excluded due to being review articles (32 articles), not reporting the necessary data for analysis (158 articles), and lacking a control group (39 articles) (Fig. 1).
Fig. 1.
Process for searching and selecting primary studies
The characteristics of the studies included in this systematic review and meta-analysis, along with the results of the risk of bias assessment, are presented in Table 1. The publication years of the articles ranged from 1993 to 2022. The studies were conducted in China [30], Egypt [31], Ethiopia [32], Iran [33, 34], India [35–40], Pakistan [41], Sri Lanka [42], Turkey [43], and the United States [44–47]. The risk of bias scores for the included articles varied from 5 to 7. Notably, three studies [36, 42, 46] reported data for more than two groups, and we analyzed these groups as independent datasets, which explains the discrepancy between the number of studies and the total pieces of evidence.
Table 1.
Characteristics of primary studies included to the present meta-analysis
| ID | First Author (Country, publication year) | Type study | Type infertility | Definition of menarche age | Number of participants with early menarche | Number of participants with late menarche | Number of participants with normal menarche | Quality score* | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early | Late | Infertile group | Fertile group | Infertile group | Fertile group | Infertile group | Fertile group | |||||
| 1 | Rich-Edwards (USA, 1993) [45] | Case-control | Primary infertility | < 11 | ≥ 17 | 190 | 3401 | 47 | 277 | 2290 | 43,040 | 6 |
| 2 |
Signorello (USA, 1997) [46] |
Case-control | Primary/secondary infertility | ≤ 11 | ≥ 13 | 9 | 16 | 27 | 48 | 14 | 25 | 5 |
| 3 |
Signorello (USA, 1997) [46] |
Case-control | Primary/secondary infertility | ≤ 11 | ≥ 13 | 5 | 16 | 27 | 48 | 15 | 25 | |
| 4 | Samarakoon (Sri Lanka, 2002) [42] | Cross-sectional | Primary infertility | < 14 | ≥ 14 | 48 | 765 | 33 | 820 | - | - | 6 |
| 5 | Samarakoon (Sri Lanka, 2002) [42] | Cross-sectional | Primary/secondary infertility | < 14 | ≥ 14 | 174 | 765 | 143 | 820 | - | - | |
| 6 | Mokhtar (Egypt, 2006) [31] | Case-control | Primary/secondary infertility | ≤ 15 | > 15 | 191 | 214 | 24 | 1 | - | - | 5 |
| 7 |
Delpishe (Iran, 2014) [33] |
Cross-sectional | Primary/secondary infertility | < 8 | > 14 | 5 | 6 | 42 | 265 | 44 | 550 | 6 |
| 8 | Gokler (Turkey, 2014) [48] | Cross-sectional | Primary/secondary infertility | ≤ 12 | ≥ 15 | 14 | 132 | 9 | 92 | 50 | 273 | 5 |
| 9 |
Saoji (India, 2014) [37] |
Case-control | Primary infertility | < 14 | > 14 | 42 | 25 | 78 | 95 | - | - | 5 |
| 10 | Yang (China, 2016) [30] | Cross-sectional | Primary infertility | ≤ 13 | ≥ 17 | 141 | 1605 | 96 | 563 | 563 | 4057 | 7 |
| 11 |
Katole (India, 2019) [35] |
Cross-sectional | Primary infertility | < 14 | > 14 | 16 | 361 | 35 | 158 | - | - | 7 |
| 12 | Khan (Pakistan, 2019) [41] | Cross-sectional | Primary/secondary infertility | ≤ 11 | ≥ 15 | 71 | 33 | 50 | 21 | 302 | 334 | 7 |
| 13 | Bayu (Ethiopia, 2020) [32] | Case-control | Primary/secondary infertility | < 14 | ≥ 14 | 30 | 113 | 63 | 75 | - | - | 5 |
| 14 |
Kamboj (India, 2022) [36] |
Cross-sectional | Primary infertility | < 12 | > 16 | 8 | 17 | 2 | 0 | 65 | 35 | 5 |
| 15 |
Kamboj (India, 2022) [36] |
Cross-sectional | Primary infertility | < 12 | > 16 | 8 | 1 | 5 | 0 | 79 | 114 | |
| 16 | SoriaContreras (USA, 2022) [44] | Cross-sectional | Primary/secondary infertility | < 12 | ≥ 15 | 37 | 144 | 19 | 97 | 153 | 615 | 7 |
| 17 |
Banerjee (India, 2023) [38] |
Cross-sectional | Primary infertility | ≤ 14 | ≥ 15 | 61 | 1014 | 74 | 846 | - | - | 6 |
| 18 |
Jenabi (Iran, 2023) [49] |
Case-control | Primary infertility | < 12 | > 14 | 35 | 56 | 28 | 19 | 137 | 125 | 5 |
| 19 |
Kataria (India, 2023) [39] |
Cross-sectional | Primary/secondary infertility | ≤ 13 | > 13 | 17 | 112 | 33 | 282 | - | - | 6 |
| 20 |
Sharma (India, 2024) [40] |
Cross-sectional | Primary/secondary infertility | < 14 | ≥ 14 | 13 | 200 | 36 | 418 | - | - | 7 |
| 21 |
Wang (USA, 2024) [47] |
Cross-sectional | Primary/secondary infertility | < 14 | ≥ 14 | 159 | 1070 | 32 | 316 | - | - | 7 |
*Quality of studies were assessed using Newcastle-Ottawa Scale Checklist
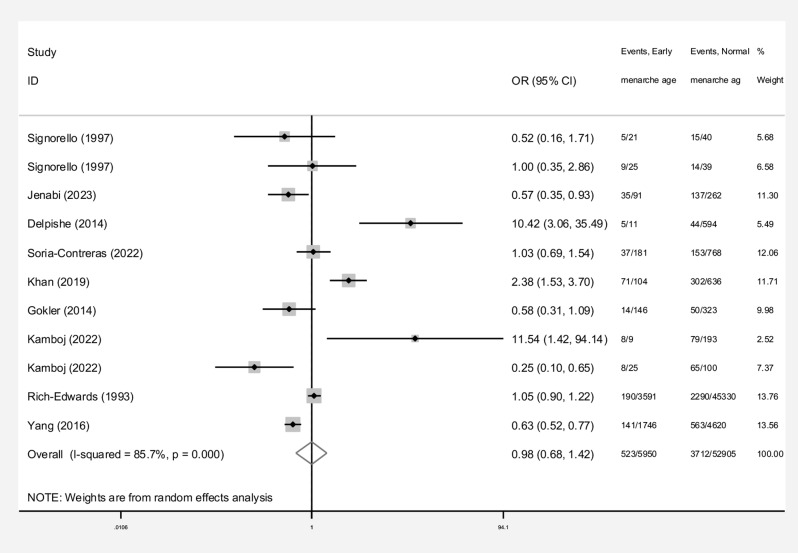
Comparison of infertility in women with late menarche vs. normal menarche age
Eleven studies compared infertility rates between women with late menarche and those with normal menarche. These studies included 1,782 women in the late menarche group and 52,905 women in the normal menarche group. Among the studies, 63.6% (7/11) reported a higher odds ratio (OR) for infertility in the late menarche group, with statistically significant differences in 27.3% (3/11). In the remaining four studies, where the OR for infertility in the late menarche group was equal to or below one, no statistically significant differences were observed (Fig. 2).
Fig. 2.
Odds ratio of infertility in women with late menarche compared to normal menarche with 95% confidence interval based on primary studies and overall estimate
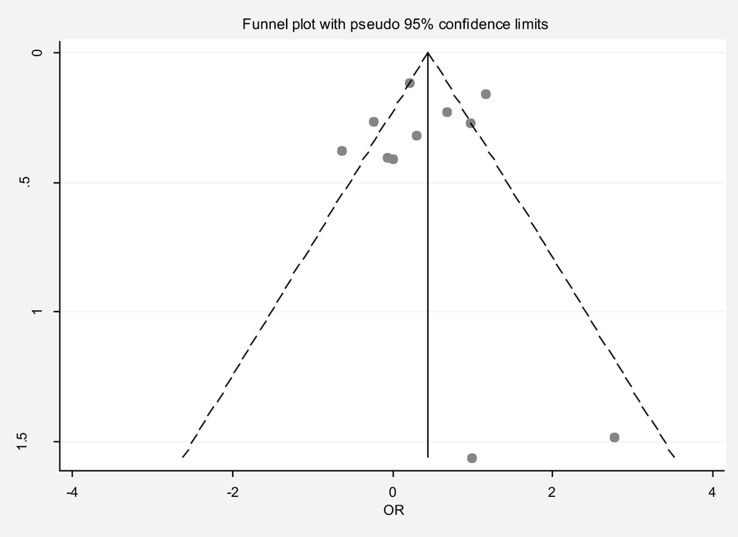
The pooled OR for infertility in the late menarche group compared to the normal menarche group was 1.44 (95% CI: 0.98, 2.10), with substantial heterogeneity (I² = 79.6%). Neither the Egger test nor the funnel plot indicated publication bias (Fig. 3). Sensitivity analysis showed that no single study significantly influenced the overall estimate (Table 2). Meta-regression analysis, considering late and early menarche criteria and type of infertility (primary/secondary), did not identify significant sources of heterogeneity (Table 3). Subgroup analysis revealed that the OR for primary infertility in the late menarche group compared to the normal menarche group was 1.98 (95% CI: 1.02, 3.85). For studies where the type of infertility was unspecified, the OR was 1.17 (95% CI: 0.71, 1.94).
Fig. 3.
Funnel plot to assess publication bias in the estimated odds ratio of infertility in women with late menarche compared to normal menarche
Table 2.
Pooled estimate of the odds ratio of infertility by menarche age
| menarche age | Number of evidence | Sample size (expose group) | Sample size (non-expose) | Pooled estimate of the odds ratio of infertility | Publication Bias total (Egger test) |
Sensitivity analysis result (Yes, No) * | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Significant impact of menarche age on the infertility (Yes, No) | β | P-value | I−square; % | p−value for Q | |||||
| Early vs. late menarche age | 21 | 11,340 | 6164 | 0.77(0.55, 1.06) | No | 0.60 | 0.590 | No | 85.6 | < 0.001 |
| Early vs. normal menarche age | 11 | 5950 | 52,905 | 0.98 (0.68, 1.42) | No | 0.63 | 0.637 | No | 85.7 | < 0.001 |
| late vs. normal menarche age | 11 | 1782 | 52,905 | 1.44(0.98, 2.10) | No | -0.27 | 0.842 | No | 79.6 | < 0.001 |
*Is there a significant difference in the impact of each of the primary studies on the overall estimate?
Table 3.
Meta-regression in order to investigate the related factors with heterogeneity on odds ratio (OR)
| Variables | OR for Early vs. late menarche | OR for Early vs. normal menarche | OR for late vs. normal menarche | |||
|---|---|---|---|---|---|---|
| β | P-value | β | P-value | β | P-value | |
| Type of infertility | 0.49 | 0.310 | -0.07 | 0.979 | -3.31 | 0.206 |
| Definition of early menarche | -0.08 | 0.574 | -0.77 | 0.525 | -0.98 | 0.432 |
| Definition of late menarche | -0.23 | 0.253 | 0.03 | 0.974 | 0.94 | 0.338 |
Comparison of infertility in women with early menarche vs. normal menarche age
Eleven studies also compared infertility rates between women with early menarche and those with normal menarche. These studies included 5,950 women in the early menarche group and 52,905 women in the normal menarche group. Of these studies, 45.5% (5/11) found a higher OR for infertility in the early menarche group, with statistically significant differences in 27.3% (3/11). In six studies where the OR was equal to or below one, significant differences were noted in three cases: Jenabi et al. (OR: 0.57, 95% CI: 0.35, 0.93), Kamboj et al. (OR: 0.25, 95% CI: 0.10, 0.65), and Yang et al. (OR: 0.63, 95% CI: 0.52, 0.77) (Fig. 4).
Fig. 4.
Odds ratio of infertility in women with early menarche compared to normal menarche with 95% confidence interval based on primary studies and overall estimate
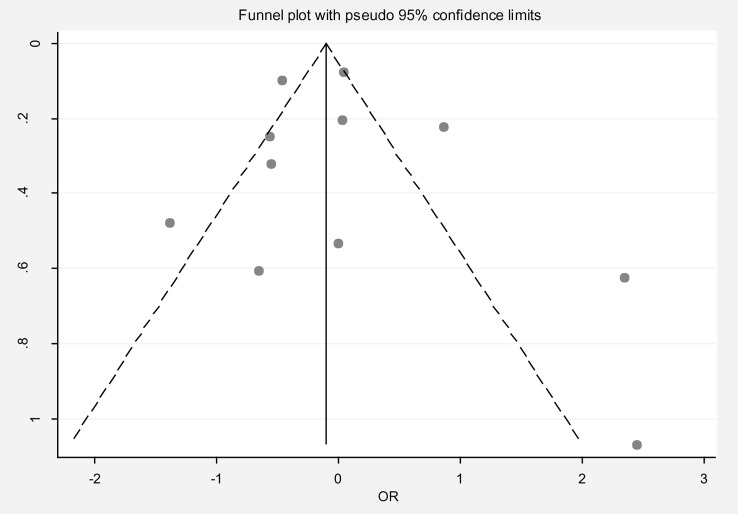
The pooled OR for infertility in the early menarche group compared to the normal menarche group was 0.98 (95% CI: 0.68, 1.42), with substantial heterogeneity (I² = 85.7%). Publication bias was not evident based on the Egger test and funnel plot (Fig. 5). Sensitivity analysis showed no significant influence of individual studies on the overall estimate (Table 2). Meta-regression analysis did not identify significant sources of heterogeneity (Table 3). Notably, there was significant variability in the definition of early menarche across studies. In one study (Delpishe et al.), early menarche was defined as occurring before eight years of age, with an OR of 10.42 (95% CI: 3.06, 35.49) for infertility compared to normal menarche.
Fig. 5.
Funnel plot to assess publication bias in the estimated odds ratio of infertility in women with early menarche compared to normal menarche
Comparison of infertility in women with early menarche vs. late menarche
Twenty-one studies compared infertility rates between women with early and late menarche. These studies included 11,340 women in the early menarche group and 6,164 women in the late menarche group. Among these studies, 38.1% (8/21) reported a higher OR for infertility in the early menarche group, with statistically significant differences in 14.3% (3/21). In contrast, 57.1% (12/21) reported a lower OR for infertility in the early menarche group, with statistically significant differences in 28.6% (6/21). One study reported an OR of one (Fig. 6).
Fig. 6.
Odds ratio of infertility in women with early menarche compared to late menarche with 95% confidence interval based on primary studies and overall estimate
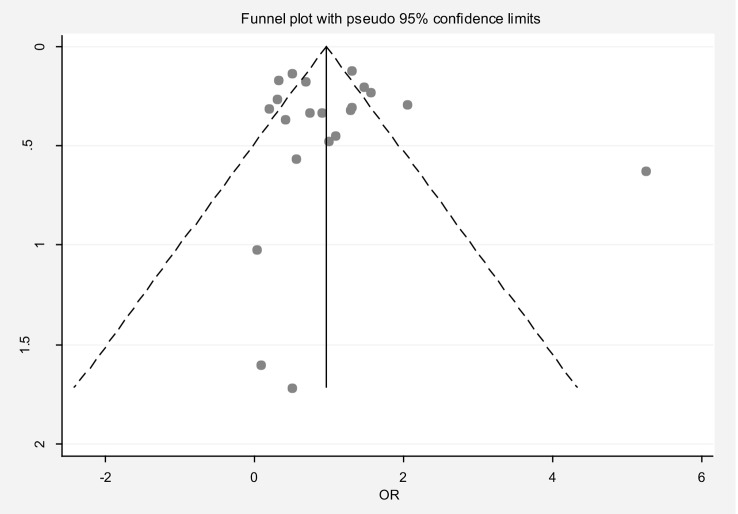
The pooled OR for infertility in the early menarche group compared to the late menarche group was 0.77 (95% CI: 0.55, 1.06), with substantial heterogeneity (I² = 85.6%). Publication bias was not evident based on the Egger test and funnel plot (Fig. 7). Sensitivity analysis confirmed that no single study significantly influenced the overall estimate (Table 2). Meta-regression analysis did not identify significant sources of heterogeneity (Table 3). Subgroup analysis revealed that the OR for primary infertility in the early menarche group compared to the late menarche group was 0.59 (95% CI: 0.36, 0.97). For studies where the type of infertility was unspecified, the OR was 0.94 (95% CI: 0.65, 1.38).
Fig. 7.
Funnel plot to assess publication bias in the estimated odds ratio of infertility in women with early menarche compared to late menarche
Discussion
In this systematic review and meta-analysis, the relationship between age at menarche and infertility was investigated. 21 pieces of evidence were analyzed, comparing infertility rates at three levels: late menarche vs. normal, early menarche vs. normal, early menarche vs. late menarche. While the overall meta-analysis results were not statistically significant for any of these comparisons, subgroup analysis revealed a significant increase in the likelihood of primary infertility associated with late menarche. This finding suggests that late menarche plays a significant role in primary infertility, with women who experience late menarche having 98% and 41% higher odds of primary infertility compared to those with normal or early menarche, respectively. Although statistical significance was not reached, the observed effect size—indicating a 44% increase in infertility odds—may still represent a potential clinical concern, particularly for reproductive health assessments. These findings suggest that late menarche likely has a greater impact on infertility than early or normal menarche in a dose-responsive manner, supporting the hypothesis that delayed menarche is a risk factor for infertility.
Cao et al. (2024) conducted a systematic review and meta-analysis examining the relationship between menstrual characteristics such as age at menarche and fertility outcomes [50]. Their findings indicated that early menarche had minimal impact on clinical pregnancy rates, while late menarche was significantly associated with reduced fertility rates. In comparison, our meta-analysis did not find a statistically significant overall association between menarche timing and infertility; however, subgroup analysis revealed a substantial increase in the likelihood of primary infertility among women with late menarche. These findings align with Cao et al.‘s conclusions, reinforcing the idea that late menarche may be a risk factor for infertility. However, while their study focused on broader fertility outcomes, our analysis specifically highlights the impact of late menarche on primary infertility, suggesting a dose-responsive relationship that warrants further investigation.
The relationship between age at menarche and infertility is not fully understood, but several mechanisms explaining how age at menarche affects infertility have been proposed. The onset of menstruation may be related to the size of the ovarian follicular pool and/or the rate of follicular atresia, which in turn predicts the decline in ovarian functional reserve in the future [51]. Anti-Müllerian hormone (AMH) is suggested as a marker of ovarian reserve, produced by pre-antral and small antral follicles, reflecting both the number of these growing small follicles and the number of primordial follicles [52–54]. Studies have shown that late menarche may be associated with lower levels of AMH [50, 55, 56]. In other words, late menarche might lead to a smaller ovarian reserve, which is a known factor in infertility [1, 2, 26]. Conversely, other studies have found that among women seeking infertility treatment, early onset of menstruation is associated with decreased ovarian reserve [25, 51, 57, 58]. Furthermore, early menarche is associated with premature and early menopause [59]. Some researchers speculate that this observed relationship might be explained by unknown prenatal exposures [10].
A lower age at first menstruation increases the incidence of diseases such as pelvic inflammatory disease, which can lead to infertility and spontaneous abortion in later years [43, 60]. In women with early menarche, 50% of their cycles are ovulatory in the first year, and nearly all cycles are ovulatory by the fifth year after menarche. In contrast, it takes about 8 to 12 years for all cycles to become ovulatory in women who experience later menarche [14, 61].
The observed relationship between age at menarche and infertility may be influenced by common underlying factors. Both lower and higher BMI compared to the normal range affect the age at menarche and infertility [7–9, 14, 61–65]. Exposure to environmental toxins, such as endocrine-disrupting chemicals (EDCs), is associated with earlier menarche and infertility markers like a lower antral follicle count (AFC) [7, 18, 66, 67]. There are also hypotheses about the influence of factors like genetics, malnutrition, intense physical exercise, psychological factors (e.g., anorexia nervosa), and chronic diseases (e.g., Autoimmune diseases) on the age at menarche and infertility [19, 68]. Nevertheless, further studies on the relationship between age at menarche and infertility and the investigation of their mechanisms are recommended.
Limitations
Our study has several limitations. One limitation is that the age at menarche was primarily self-reported based on recall. However, previous studies have shown that individuals can recall the age at menarche with reasonable accuracy [63, 69], and self-reported age at menarche for middle-aged women has a moderate correlation with recorded data from adolescence [70]. A key limitation of this study is the reliance on self-reported infertility, which may be subject to recall bias or incomplete disclosure of reproductive history. Additionally, we did not exclude studies that involved populations using oral contraceptives, assisted reproductive technologies, or those with a known history of infertility. While such factors could influence infertility outcomes, excluding these studies would have significantly reduced the available data and compromised the study’s generalizability. Instead, we applied a standardized definition of infertility—failure to conceive after one year of unprotected intercourse—to ensure consistency across included studies. Publication biases and language inclusion criteria might have limited the evaluated studies, but these factors were statistically accounted for. The primary limitation of any meta-analysis is the quality of the primary studies, as some had limited data and could not be included in the analysis.
Conclusion
Although the overall meta-analysis did not yield statistically significant results, subgroup analysis identified a notable association between late menarche and primary infertility. Women with late menarche had 98% and 41% higher odds of primary infertility compared to those with normal and early menarche, respectively. While statistical significance was not reached, the observed 44% increase in infertility odds suggests a potential clinical concern. These findings highlight late menarche as a possible risk factor for infertility, supporting a dose-responsive relationship. Further research is needed to clarify this association and its implications for reproductive health assessments and early intervention strategies.
Acknowledgements
Not applicable.
Abbreviations
- BMI
Body mass index
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- AMH
Anti-Müllerian hormone
- OR
Odds ratio
- CI
Confidence interval
- NOS
Newcastle-Ottawa Scale
Author contributions
MM: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Visualization, Supervision, Project administration, Writing - Original Draft, Writing – review & editing. AHB: Methodology, Validation, Investigation, Resources, Data curation, Supervision, Project administration, Writing – review & editing. SMH: Methodology, Investigation, Resources, Data curation, Writing – review & editing. MGT: Conceptualization, Validation, Supervision, Writing – review & editing. SKS: Methodology, Investigation, Resources, Data curation, Writing – review & editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carson SA, Kallen AN. Diagnosis and management of infertility: A review. JAMA. 2021;326(1):65–76. 10.1001/jama.2021.4788. Epub 2021/07/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker MH, Tobler KJ, Female, Infertility. Statpearls. Treasure Island (FL) ineligible companies. Disclosure: Kyle Tobler declares no relevant financial relationships with ineligible companies.: StatPearls publishing. Copyright © 2024. StatPearls Publishing LLC; 2024.
- 3.Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc. 1956;161(9):855–60. 10.1001/jama.1956.02970090081016. Epub 1956/06/30. [DOI] [PubMed] [Google Scholar]
- 4.Farquhar CM, Bhattacharya S, Repping S, Mastenbroek S, Kamath MS, Marjoribanks J, et al. Female subfertility. Nat Rev Dis Primers. 2019;5(1):7. 10.1038/s41572-018-0058-8. Epub 2019/01/27. [DOI] [PubMed] [Google Scholar]
- 5.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. 10.1371/journal.pmed.1001356. Epub 2012/12/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and Treatment-Seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–12. 10.1093/humrep/dem046. Epub 2007/03/23. [DOI] [PubMed] [Google Scholar]
- 7.Shirazi TN, Rosinger AY. Reproductive health disparities in the USA: Self-Reported race/ethnicity predicts age of menarche and live birth ratios, but not infertility. J Racial Ethnic Health Disparities. 2021;8(1):33–46. 10.1007/s40615-020-00752-4. [DOI] [PubMed] [Google Scholar]
- 8.Frisch RE. The right weight: body fat, menarche and ovulation. Baillieres Clin Obstet Gynaecol. 1990;4(3):419–39. 10.1016/s0950-3552(05)80302-5. Epub 1990/09/01. [DOI] [PubMed] [Google Scholar]
- 9.Crosignani PG, Colombo M, Vegetti W, Somigliana E, Gessati A, Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003;18(9):1928–32. 10.1093/humrep/deg367. Epub 2003/08/19. [DOI] [PubMed] [Google Scholar]
- 10.Guldbrandsen K, Håkonsen LB, Ernst A, Toft G, Lyngsø J, Olsen J, et al. Age of menarche and time to pregnancy. Hum Reprod. 2014;29(9):2058–64. 10.1093/humrep/deu153. Epub 2014/07/26. [DOI] [PubMed] [Google Scholar]
- 11.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668–93. 10.1210/er.2002-0019. Epub 2003/10/23. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Wang YY, Zhang Y, Zhang HG, Yang Y, He Y, et al. The influence of age at menarche, menstrual cycle length and bleeding duration on time to pregnancy: A large prospective cohort study among rural Chinese women. BJOG. 2017;124(11):1654–62. 10.1111/1471-0528.14469. Epub 2017/01/28. [DOI] [PubMed] [Google Scholar]
- 13.Acog Committee Opinion No. 651: Menstruation in Girls and Adolescents: Using the Menstrual Cycle as a Vital Sign. Obstet Gynecol. (2015) 126(6):e143-e6. Epub 2015/11/26. 10.1097/aog.0000000000001215 [DOI] [PubMed]
- 14.Lacroix AE, Gondal H, Shumway KR, Langaker MD, Physiology. Menarche. Statpearls. Treasure Island (FL) ineligible companies. Disclosure: Hurria Gondal declares no relevant financial relationships with ineligible companies. Disclosure: Karlie Shumway declares no relevant financial relationships with ineligible companies. Disclosure: Michelle Langaker declares no relevant financial relationships with ineligible companies. StatPearls Publishing; 2024. Copyright © 2024.
- 15.De Sanctis V, Rigon F, Bernasconi S, Bianchin L, Bona G, Bozzola M, et al. Age at menarche and menstrual abnormalities in adolescence: does it matter?? The evidence from a large survey among italian secondary schoolgirls. Indian J Pediatr. 2019;86(Suppl 1):34–41. 10.1007/s12098-018-2822-x. Epub 2019/01/11. [DOI] [PubMed] [Google Scholar]
- 16.Lee HS. Why should we be concerned about early menarche?? Clin Exp Pediatr. 2021;64(1):26–7. 10.3345/cep.2020.00521. Epub 2020/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin YC, Yen HR, Wang CH, Liao YC, Lin RT. Trends in age at menarche from 1943 through 1989 in Taiwan: A retrospective Population-Based analysis. Pediatr Neonatol. 2024;65(1):64–70. 10.1016/j.pedneo.2023.07.001. Epub 2023/08/13. [DOI] [PubMed] [Google Scholar]
- 18.Park O, Park JT, Chi Y, Kwak K. Association of phthalates and early menarche in Korean adolescent girls from Korean National environmental health survey (Konehs) 2015–2017. Ann Occup Environ Med. 2021;33:e4. 10.35371/aoem.2021.33.e4. Epub 2021/11/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warp ML, Grindstad T, Magnus MC, Page CM, Håberg SE, Morken NH, et al. Early or late menarche is associated with reduced fecundability in the Norwegian mother, father and child cohort study. Hum Reprod. 2024;39(4):812–21. 10.1093/humrep/deae011. [DOI] [PubMed] [Google Scholar]
- 20.Menarche M, Breast Cancer Risk. Individual participant Meta-Analysis, including 118 964 women with breast Cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–51. 10.1016/s1470-2045(12)70425-4. Epub 2012/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94(12):4953–60. 10.1210/jc.2009-1789. Epub 2009/11/03. [DOI] [PubMed] [Google Scholar]
- 22.Kaltiala-Heino R, Kosunen E, Rimpelä M. Pubertal timing, sexual behaviour and Self-Reported depression in middle adolescence. J Adolesc. 2003;26(5):531–45. 10.1016/s0140-1971(03)00053-8. Epub 2003/09/16. [DOI] [PubMed] [Google Scholar]
- 23.Downing J, Bellis MA. Early pubertal onset and its relationship with sexual risk taking, substance use and Anti-Social behaviour: A preliminary Cross-Sectional study. BMC Public Health. 2009;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox KM, Magaziner J, Sherwin R, Scott JC, Plato CC, Nevitt M, et al. Reproductive correlates of bone mass in elderly women. Study of osteoporotic fractures research group. J Bone Min Res. 1993;8(8):901–8. 10.1002/jbmr.5650080802. Epub 1993/08/01. [DOI] [PubMed] [Google Scholar]
- 25.Wesselink AK, Wise LA, Hatch EE, Rothman KJ, Mikkelsen EM, Stanford JB, et al. Menstrual cycle characteristics and fecundability in a North American preconception cohort. Ann Epidemiol. 2016;26(7):482–7. 10.1016/j.annepidem.2016.05.006..e1. Epub 2016/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Zhong C, Liang H, Yang Y, Zhang O, Gao E, et al. The relationship between age at menarche and infertility among Chinese rural women. Eur J Obstet Gynecol Reprod Biol. 2015;194:68–72. 10.1016/j.ejogrb.2015.08.016. Epub 2015/09/04. [DOI] [PubMed] [Google Scholar]
- 27.Adamson PC, Krupp K, Freeman AH, Klausner JD, Reingold AL, Madhivanan P. Prevalence & correlates of primary infertility among young women in Mysore, India. Indian J Med Res. 2011;134(4):440–6. Epub 2011/11/18. [PMC free article] [PubMed] [Google Scholar]
- 28.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Bmj (2021) 372:n71. Epub 20210329. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed]
- 29.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in Meta-Analyses. Eur J Epidemiol. 2010;25(9):603–5. 10.1007/s10654-010-9491-z. Epub 2010/07/24. [DOI] [PubMed] [Google Scholar]
- 30.Yang Fen YF, Li Lin LL, Chen JianPing CJ, Liu XiaoQin LX, Zhong ChunLi ZC, Yang Yuan YY et al. Couple’s Infertility in Relation to Male Smoking in a Chinese Rural Area. (2017). [DOI] [PMC free article] [PubMed]
- 31.Mokhtar S, Hassan HA, Mahdy N, Elkhwsky F, Shehata G. Risk factors for primary and secondary female infertility in Alexandria: A Hospital-Based Case-Control study. J Med Res Inst. 2006;27:251–61. [Google Scholar]
- 32.Bayu D, Egata G, Kefale B, Jemere T. Determinants of Infertility among Married Women Attending Dessie Referral Hospital and Dr. Misganaw Gynecology and Obstetrics Clinic, Dessie, Ethiopia. Int J Reprod Med (2020) 2020:1540318. Epub 2020/04/14. 10.1155/2020/1540318 [DOI] [PMC free article] [PubMed]
- 33.Delpishe A, Direkvand Moghadam A, Moradi z, Mir Moghadam N. Aspects of epidemiology of infertility in Ilam in 2013. Iran J Obstet Gynecol Infertility. 2014;17(98):8–14. 10.22038/ijogi.2014.2831. [Google Scholar]
- 34.Jenabi E, Ayubi E, Khazaei S, Abdoli S. Lifestyle among fertile and infertile women: A Case-Control study in the West of Iran. Curr Women’s Health Reviews. 2023;19(4):57–62. [Google Scholar]
- 35.Katole A, Saoji AV. Prevalence of primary infertility and its associated risk factors in urban population of central India: A Community-Based Cross-Sectional study. Indian J Community Med. 2019;44(4):337–41. 10.4103/ijcm.IJCM_7_19. Epub 2019/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamboj N, Saraswathy KN, Babu N, Puri M, Sachdeva MP, Mahajan C. Infertility and obesity: A Cross-Sectional study in North Indian women. Coll Antropol. 2022;46(2):97–103. [Google Scholar]
- 37.Saoji AV. Primary infertility problems among female have been a source of concern in India lately. Innovative J Med Health Sci. 2014;4(1):332–40. [Google Scholar]
- 38.Banerjee AB, Jain P, Choudhary K, Banerjee A. Primary infertility: A Rural-Based study of associated risk factors in North-West part of India. Int J Infertility Fetal Med. 2023;14(1):42–6. [Google Scholar]
- 39.Kataria D, Rani B, Punia A, Jha SK, Narendran M, Singh J. Reproductive risk factors associated with female infertility in Sonepat district of Haryana: A community based Cross-Sectional study. J Hum Reprod Sci. 2023;16(3):204–11. 10.4103/jhrs.jhrs_82_23. Epub 2023/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma R, Bakshi H, Patel P, Patel B, Gajjar S, Dave R, et al. Burden of infertility, its risk factors, perceptions and challenges faced by women of Peri-Urban community from Ahmedabad City: mixed method study. Indian J Community Med. 2024;49(5):687–94. 10.4103/ijcm.ijcm_428_23. Epub 2024/10/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan HL, Bhatti S, Suhail S, Gul R, Awais A, Hamayun H, et al. Antral follicle count (Afc) and serum Anti-Müllerian hormone (Amh) are the predictors of natural fecundability have similar trends irrespective of fertility status and menstrual characteristics among fertile and infertile women below the age of 40 years. Reprod Biol Endocrinol. 2019;17(1):20. 10.1186/s12958-019-0464-0. Epub 2019/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samarakoon S, Rajapaksa L, Seneviratne H. Prevalence of Primary and Secondary Infertility in the Colombo District. (2002).
- 43.Gokler ME, Unsal A, Arslantas D. The prevalence of infertility and loneliness among women aged 18–49 years who are living in Semi-Rural areas in Western Turkey. Int J Fertil Steril. 2014;8(2):155–62. Epub 2014/08/02. [PMC free article] [PubMed] [Google Scholar]
- 44.Soria-Contreras DC, Perng W, Rifas-Shiman SL, Hivert MF, Oken E, Chavarro JE. History of infertility and pregnancy outcomes in project Viva: A prospective study. BMC Pregnancy Childbirth. 2022;22(1):549. 10.1186/s12884-022-04885-8. Epub 2022/07/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171–7. 10.1016/0002-9378(94)90465-0. Epub 1994/07/01. [DOI] [PubMed] [Google Scholar]
- 46.Signorello LB, Harlow BL, Cramer DW, Spiegelman D, Hill JA. Epidemiologic determinants of endometriosis: A Hospital-Based Case-Control study. Ann Epidemiol. 1997;7(4):267–741. 10.1016/s1047-2797(97)00017-3. Epub 1997/05/01. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Lai YH, Huang SY, Liu YD, Chen SL. Combined impact of sleep and obesity on female infertility in the Nhanes 2017–2020. BMC Womens Health. 2024;24(1):315. 10.1186/s12905-024-03164-2. Epub 2024/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gokler ME, Unsal A, Arslantas D. The prevalence of infertility and loneliness among women aged 18–49 years who are living in Semi-Rural areas in Western Turkey. Int J Fertility Steril. 2014;8(2):155. [PMC free article] [PubMed] [Google Scholar]
- 49.Ensiyeh J, Erfan A, Salman K, Sara A. Lifestyle among fertile and infertile women: A Case-Control study in the West of Iran. Curr Women`s Health Reviews. 2023;19(4):57–62. 10.2174/1573404819666220930143303. [Google Scholar]
- 50.Cao Y, Zhao X, Dou Z, Gong Z, Wang B, Xia T. The correlation between menstrual characteristics and fertility in women of reproductive age: A systematic review and Meta-Analysis. Fertil Steril. 2024;122(5):918–27. 10.1016/j.fertnstert.2024.06.016. Epub 20240625. [DOI] [PubMed] [Google Scholar]
- 51.Weghofer A, Kim A, Barad DH, Gleicher N. Age at menarche: A predictor of diminished ovarian function?? Fertil Steril. 2013;100(4):1039–43. 10.1016/j.fertnstert.2013.05.042. Epub 2013/07/03. [DOI] [PubMed] [Google Scholar]
- 52.La Marca A, Volpe A. Anti-Müllerian hormone (Amh) in female reproduction: is measurement of Circulating Amh a useful tool?? Clin Endocrinol (Oxf). 2006;64(6):603–10. 10.1111/j.1365-2265.2006.02533.x. Epub 2006/05/23. [DOI] [PubMed] [Google Scholar]
- 53.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and Cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. 10.1093/molehr/gah015. Epub 2004/01/27. [DOI] [PubMed] [Google Scholar]
- 54.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–5. 10.1016/j.fertnstert.2010.04.006. Epub 2010/06/05. [DOI] [PubMed] [Google Scholar]
- 55.Bragg JM, Kuzawa CW, Agustin SS, Banerjee MN, McDade TW. Age at menarche and parity are independently associated with Anti-Müllerian hormone, a marker of ovarian reserve, in Filipino young adult women. Am J Hum Biol. 2012;24(6):739–45. 10.1002/ajhb.22309. Epub 2012/08/24. [DOI] [PubMed] [Google Scholar]
- 56.Kerkhof GF, Leunissen RW, Willemsen RH, de Jong FH, Visser JA, Laven JS, et al. Influence of preterm birth and small birth size on serum Anti-Müllerian hormone levels in young adult women. Eur J Endocrinol. 2010;163(6):937–44. 10.1530/eje-10-0528. Epub 2010/10/05. [DOI] [PubMed] [Google Scholar]
- 57.Vogiatzi P, Pouliakis A, Bettocchi S, Daskalakis G, Vrantza T, Siristatidis C. Age at menarche and clinical outcomes following medically assisted reproduction (Mar): A cohort study. Gynecol Endocrinol. 2019;35(5):448–52. 10.1080/09513590.2018.1538344. Epub 2019/02/19. [DOI] [PubMed] [Google Scholar]
- 58.Sadrzadeh S, Klip WA, Broekmans FJ, Schats R, Willemsen WN, Burger CW, et al. Birth weight and age at menarche in patients with polycystic ovary syndrome or diminished ovarian reserve, in a retrospective cohort. Hum Reprod. 2003;18(10):2225–30. 10.1093/humrep/deg409. Epub 2003/09/26. [DOI] [PubMed] [Google Scholar]
- 59.Mishra GD, Pandeya N, Dobson AJ, Chung HF, Anderson D, Kuh D, et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod. 2017;32(3):679–86. 10.1093/humrep/dew350. Epub 2017/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Temporal Relationships between Ovulation and Defined Changes in the Concentration of Plasma Estradiol-17 Beta, Hormone L, Hormone F-S, Progesterone I, Probit. Analysis. World Health Organization, Task Force on Methods for the Determination of the Fertile Period, Special Programme of Research, Development and Research Training in Human Reproduction. Am J Obstet Gynecol (1980) 138(4):383– 90. Epub 1980/10/15. [PubMed]
- 61.Hickey M, Balen A. Menstrual disorders in adolescence: investigation and management. Hum Reprod Update. 2003;9(5):493–504. 10.1093/humupd/dmg038. Epub 2003/12/03. [DOI] [PubMed] [Google Scholar]
- 62.Yen SS, Rebar R, Vandenberg G, Ehara Y, Siler T. Pituitary gonadotrophin responsiveness to synthetic Lrf in subjects with normal and abnormal Hypothalamic-Pituitary-Gonadal Axis. J Reprod Fertil Suppl. 1973;20(0):137–61. Epub 1973/12/01. [PubMed] [Google Scholar]
- 63.Gong TT, Wang YL, Ma XX. Age at menarche and endometrial Cancer risk: A Dose-Response Meta-Analysis of prospective studies. Sci Rep. 2015;5:14051. 10.1038/srep14051. Epub 2015/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pierce MB, Leon DA. Age at menarche and adult Bmi in the Aberdeen children of the 1950s cohort study. Am J Clin Nutr. 2005;82(4):733–9. 10.1093/ajcn/82.4.733. Epub 2005/10/08. [DOI] [PubMed] [Google Scholar]
- 65.Baker ER. Body weight and the initiation of puberty. Clin Obstet Gynecol. 1985;28(3):573–9. 10.1097/00003081-198528030-00013. Epub 1985/09/01. [DOI] [PubMed] [Google Scholar]
- 66.Buttke DE, Sircar K, Martin C. Exposures to Endocrine-Disrupting chemicals and age of menarche in adolescent girls in Nhanes (2003–2008). Environ Health Perspect. 2012;120(11):1613–8. 10.1289/ehp.1104748. Epub 2012/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Messerlian C, Souter I, Gaskins AJ, Williams PL, Ford JB, Chiu YH, et al. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Hum Reprod. 2016;31(1):75–83. 10.1093/humrep/dev292. Epub 2015/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Komura H, Miyake A, Chen CF, Tanizawa O, Yoshikawa H. Relationship of age at menarche and subsequent fertility. Eur J Obstet Gynecol Reprod Biol. 1992;44(3):201–3. 10.1016/0028-2243(92)90099-k. Epub 1992/05/13. [DOI] [PubMed] [Google Scholar]
- 69.Bean JA, Leeper JD, Wallace RB, Sherman BM, Jagger H. Variations in the reporting of menstrual histories. Am J Epidemiol. 1979;109(2):181–5. 10.1093/oxfordjournals.aje.a112673. Epub 1979/02/01. [DOI] [PubMed] [Google Scholar]
- 70.Cooper R, Blell M, Hardy R, Black S, Pollard TM, Wadsworth ME, et al. Validity of age at menarche Self-Reported in adulthood. J Epidemiol Community Health. 2006;60(11):993–7. 10.1136/jech.2005.043182. Epub 2006/10/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.