Abstract
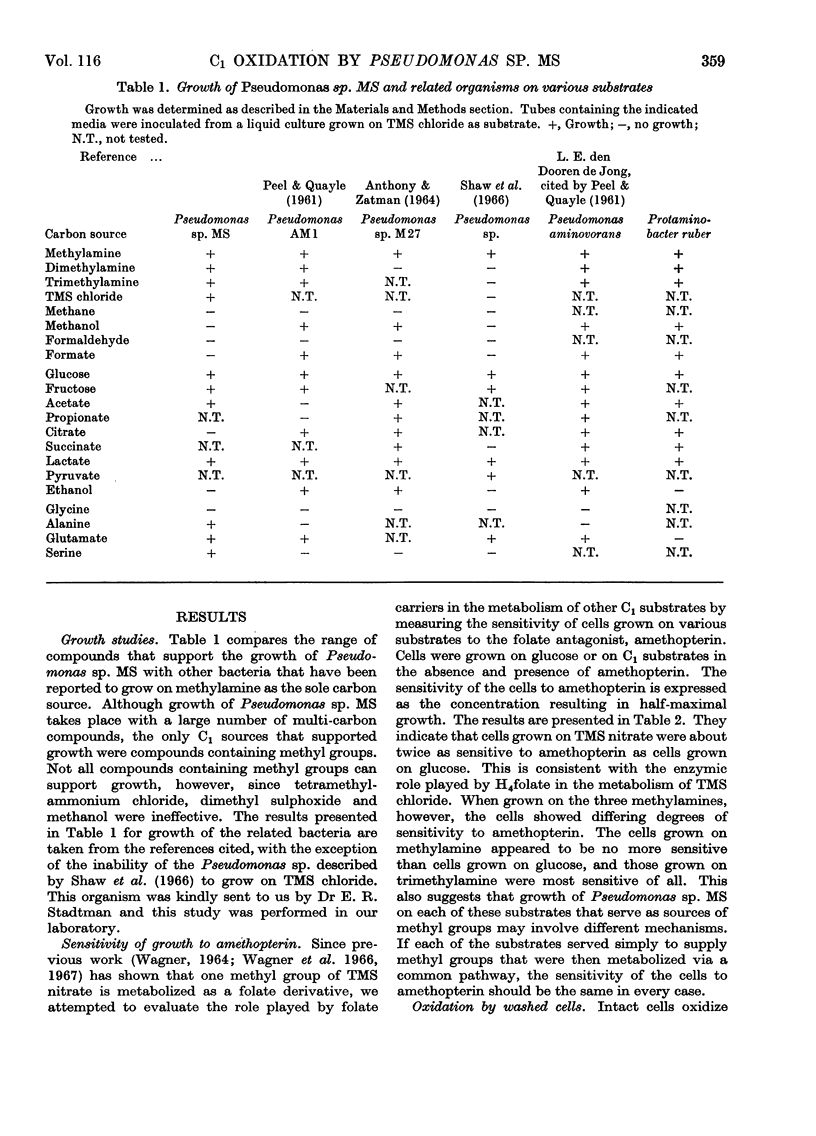
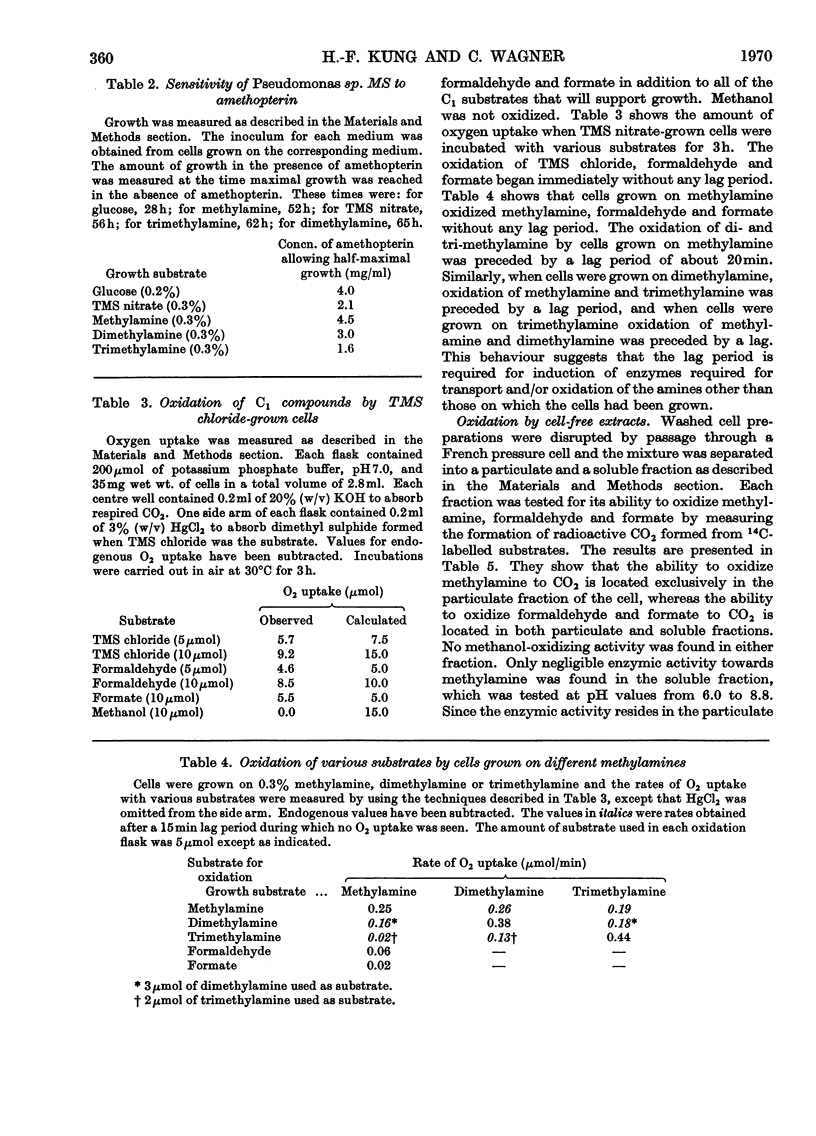
Pseudomonas sp. MS is capable of growth on a number of compounds containing only C1 groups. They include trimethylsulphonium salts, methylamine, dimethylamine and trimethylamine. Although formaldehyde and formate will not support growth they are rapidly oxidized by intact cells. Methanol neither supports growth nor is oxidized. A particulate fraction of the cell oxidizes methylamine to carbon dioxide in the absence of any external electron acceptor. Formaldehyde and formate are more slowly oxidized to carbon dioxide by the particulate fraction, although they do not appear to be free intermediates in the oxidation of methylamine. Soluble NAD-linked formaldehyde dehydrogenase and formate dehydrogenase are also present. The particulate methylamine oxidase is induced by growth on methylamine, dimethylamine and trimethylamine, whereas the soluble formaldehyde dehydrogenase and formate dehydrogenase are induced by trimethylsulphonium nitrate as well as the aforementioned amines.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 1. Isolation and properties of Pseudomonas sp. M27. Biochem J. 1964 Sep;92(3):609–614. doi: 10.1042/bj0920609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER S. N., KALLIO R. E. Bacterial degradation of methylurea. Arch Biochem Biophys. 1958 Aug;76(2):295–305. doi: 10.1016/0003-9861(58)90155-3. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEDA T., ROXBURGH M. J. A methanol-utilizing bacterium. II. Studies on the pathway of methanol assimilation. Can J Microbiol. 1959 Apr;5(2):187–195. doi: 10.1139/m59-023. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leadbetter E. R., Gottlieb J. A. On methylamine assimilation in a bacterium. Arch Mikrobiol. 1967;59(1):211–217. doi: 10.1007/BF00406334. [DOI] [PubMed] [Google Scholar]
- PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J. 1961 Dec;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- SELIGSON D., SELIGSON H. A microdiffusion method for the determination of nitrogen liberated as ammonia. J Lab Clin Med. 1951 Aug;38(2):324–330. [PubMed] [Google Scholar]
- Shaw W. V., Tsai L., Stadtman E. R. The enzymatic synthesis of N-methylglutamic acid. J Biol Chem. 1966 Feb 25;241(4):935–945. [PubMed] [Google Scholar]
- Wagner C., Lusty S. M., Jr, Kung H. F., Rogers N. L. Preparation and properties of trimethylsulfonium-tetrahydrofolate methyltransferase. J Biol Chem. 1967 Mar 25;242(6):1287–1293. [PubMed] [Google Scholar]
- Wagner C., Lusty S. M., Jr, Kung H. F., Rogers N. L. Trimethylsulfonium-tetrahydrofolate methyltransferase, a novel enzyme in the utilization of 1 carbon units. J Biol Chem. 1966 Apr 25;241(8):1923–1925. [PubMed] [Google Scholar]


