Abstract
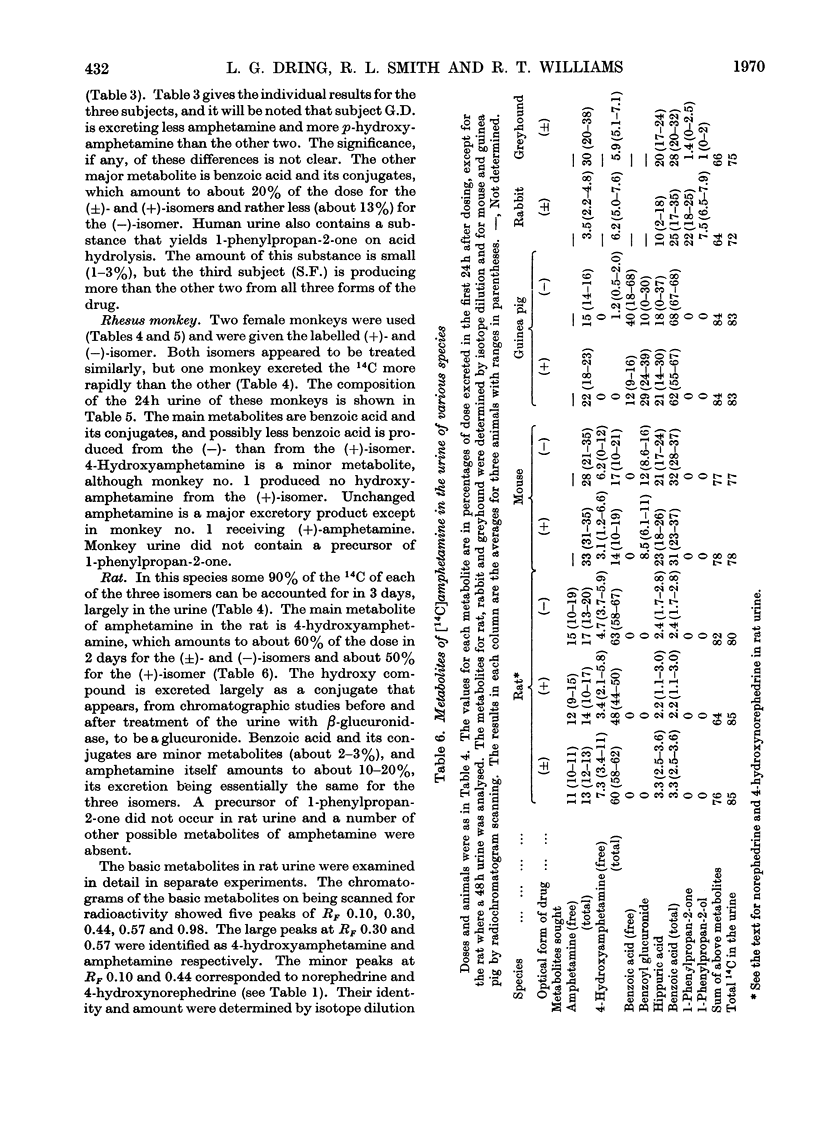
1. The fate of [14C]amphetamine in man, rhesus monkey, greyhound, rat, rabbit, mouse and guinea pig has been studied. 2. In three men receiving orally 5mg each (about 0.07mg/kg), about 90% of the 14C was excreted in the urine in 3–4 days. About 60–65% of the 14C was excreted in 1 day, 30% as unchanged drug, 21% as total benzoic acid and 3% as 4-hydroxyamphetamine. 3. In two rhesus monkeys (dose 0.66mg/kg), the metabolites excreted in 24h were similar to those in man except that there was little 4-hydroxyamphetamine. 4. In greyhounds receiving 5mg/kg intraperitoneally the metabolites were similar in amount to those in man. 5. Rabbits receiving 10mg/kg orally differed from all other species. They excreted little unchanged amphetamine (4% of dose) and 4-hydroxyamphetamine (6%). They excreted in 24h mainly benzoic acid (total 25%), an acid-labile precursor of 1-phenylpropan-2-one (benzyl methyl ketone) (22%) and conjugated 1-phenylpropan-2-ol (benzylmethylcarbinol) (7%). 6. Rats receiving 10mg/kg orally also differed from other species. The main metabolite (60% of dose) was conjugated 4-hydroxyamphetamine. Minor metabolites were amphetamine (13%), N-acetylamphetamine (2%), norephedrine (0.3%) and 4-hydroxynorephedrine (0.3%). 7. The guinea pig receiving 5mg/kg excreted only benzoic acid and its conjugates (62%) and amphetamine (22%). 8. The mouse receiving 10mg/kg excreted amphetamine (33%), 4-hydroxyamphetamine (14%) and benzoic acid and its conjugates (31%). 9. Experiments on the precursor of 1-phenylpropan-2-one occurring in rabbit urine suggest that it might be the enol sulphate of the ketone. A very small amount of the ketone (1–3%) was also found in human and greyhound urine after acid hydrolysis.
Full text
PDF





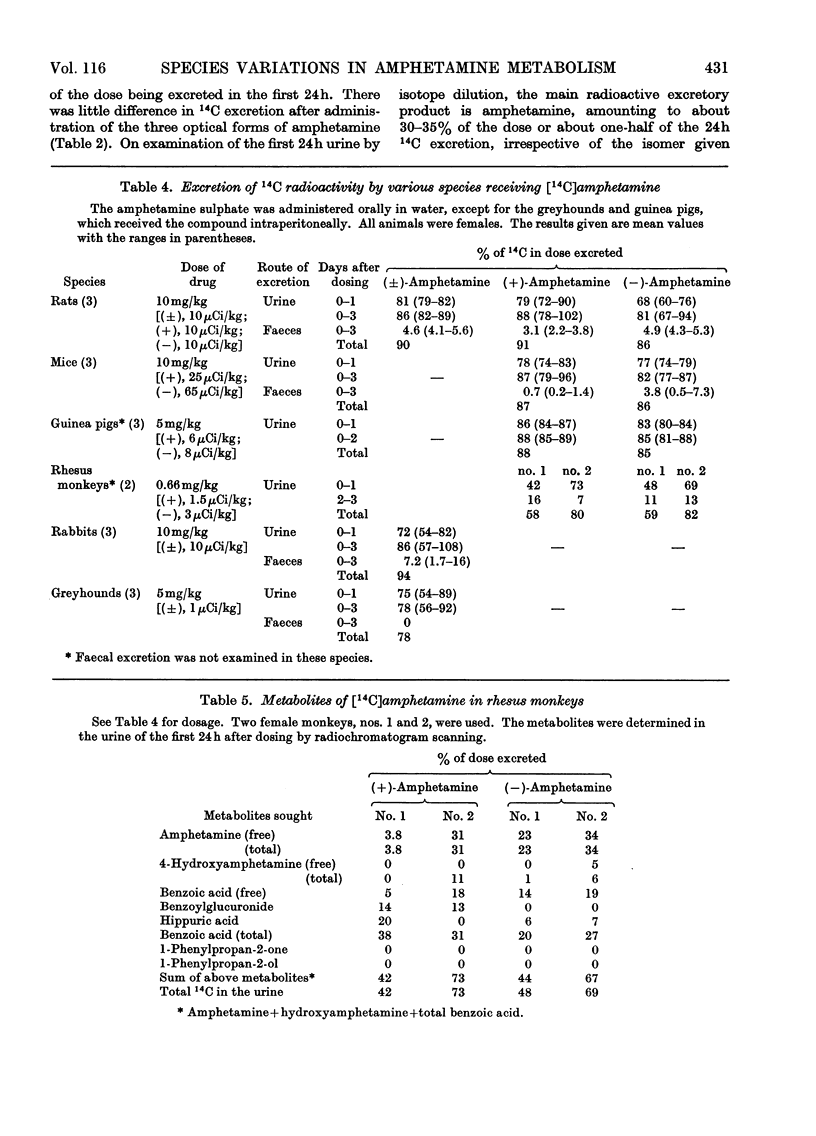



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEVA J. J. METABOLISM OF TRANYLEYPROMINE-C14 AND DL AMPHETAMINE-C14 IN THE RAT. J Med Chem. 1963 Nov;6:621–624. doi: 10.1021/jm00342a001. [DOI] [PubMed] [Google Scholar]
- ASATOOR A. M., GALMAN B. R., JOHNSON J. R., MILNE M. D. THE EXCRETION OF DEXAMPHETAMINE AND ITS DERIVATIVES. Br J Pharmacol Chemother. 1965 Feb;24:293–300. doi: 10.1111/j.1476-5381.1965.tb02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AXELROD J. Studies on sympathomimetic amines. II. The biotransformation and physiological disposition of d-amphetamine, d-p-hydroxyamphetamine and d-methamphetamine. J Pharmacol Exp Ther. 1954 Mar;110(3):315–326. [PubMed] [Google Scholar]
- AXELROD J. The enzymatic deamination of amphetamine (benzedrine). J Biol Chem. 1955 Jun;214(2):753–763. [PubMed] [Google Scholar]
- Beckett A. H., Rowland M. Urinary excretion kinetics of amphetamine in man. J Pharm Pharmacol. 1965 Oct;17(10):628–639. doi: 10.1111/j.2042-7158.1965.tb07575.x. [DOI] [PubMed] [Google Scholar]
- Bridges J. W., Davies D. S., Williams R. T. The fate of ethyltin and diethyltin derivatives in the rat. Biochem J. 1967 Dec;105(3):1261–1267. doi: 10.1042/bj1051261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges J. W., Kibby M. R., Williams R. T. The structure of the glucuronide of sulphadimethoxine formed in man. Biochem J. 1965 Sep;96(3):829–836. doi: 10.1042/bj0960829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRY A. S., POWELL H. Paper chromatographic examination of the alkaloid extract in toxicology. Nature. 1954 Jun 12;173(4415):1143–1144. doi: 10.1038/1731143b0. [DOI] [PubMed] [Google Scholar]
- Dring L. G., Smith R. L., Williams R. T. A precursor of benzyl methyl ketone in amphetamine urine. Biochem J. 1968 Sep;109(2):10P–11P. doi: 10.1042/bj1090010pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dring L. G., Smith R. L., Williams R. T. The fate of amphetamine in man and other mammals. J Pharm Pharmacol. 1966 Jun;18(6):402–404. doi: 10.1111/j.2042-7158.1966.tb07896.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., BURKHALTER A., LADOU J. A fluorometric method for the determination of hippuric acid. J Lab Clin Med. 1961 May;57:813–818. [PubMed] [Google Scholar]
- Ellison T., Gutzait L., Van Loon E. J. The comparative metabolism of d-amphetamine-C14 in the rat, dog and monkey. J Pharmacol Exp Ther. 1966 Jun;152(3):383–387. [PubMed] [Google Scholar]
- FEWSTER M. E., HALL D. A. Application of buffered solvent systems to the detection of aromatic acids by paper partition chromatography. Nature. 1951 Jul 14;168(4263):78–79. doi: 10.1038/168078a0. [DOI] [PubMed] [Google Scholar]
- Fairchild M. D., Alles G. A. The central locomotor stimulatory acitivity and acute toxicity of the ephedrine and norephedrine isomers in mice. J Pharmacol Exp Ther. 1967 Oct;158(1):135–139. [PubMed] [Google Scholar]
- GAFFNEY G. W., SCHREIER K., DIFERRANTE N., ALTMAN K. I. The quantitative determination of hippuric acid. J Biol Chem. 1954 Feb;206(2):695–698. [PubMed] [Google Scholar]
- GOLDSTEIN M., CONTRERA J. F. The substrate specificity of phenylamine-beta-hydroxylase. J Biol Chem. 1962 Jun;237:1898–1902. [PubMed] [Google Scholar]
- GOLDSTEIN M., MCKEREGHAN M. R., LAUBER E. THE STEREOSPECIFICITY OF THE ENZYMATIC AMPHETAMINE BETA-HYDROXYLATION. Biochim Biophys Acta. 1964 Jul 8;89:191–193. doi: 10.1016/0926-6569(64)90124-5. [DOI] [PubMed] [Google Scholar]
- Goldstein M., Anagnoste B. The conversion in vivo of D-amphetamine to (+)-p-hydroxynorephedrine. Biochim Biophys Acta. 1965 Aug 24;107(1):166–168. doi: 10.1016/0304-4165(65)90412-5. [DOI] [PubMed] [Google Scholar]
- Gunne L. M. The urinary output of d- and l-amphetamine in man. Biochem Pharmacol. 1967 May;16(5):863–869. doi: 10.1016/0006-2952(67)90059-7. [DOI] [PubMed] [Google Scholar]
- Gunne L., Galland L. Stereoselective metabolism of amphetamine. Biochem Pharmacol. 1967 Jul 7;16(7):1374–1377. doi: 10.1016/0006-2952(67)90170-0. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. W., MOORE S., STEIN W. H. A chromatographic investigation of pancreatic ribonuclease. J Biol Chem. 1953 Feb;200(2):493–506. [PubMed] [Google Scholar]
- Iversen L. L., Glowinski J., Axelrod J. The physiologic disposition and metabolism of norepinephrine in immunosympathectomized animals. J Pharmacol Exp Ther. 1966 Feb;151(2):273–284. [PubMed] [Google Scholar]
- KARIYONE T., HASHIMOTO Y., KIMURA M. Paper partition chromatography of alcohols using the potassium xanthogenates. Nature. 1951 Sep 22;168(4273):511–511. doi: 10.1038/168511a0. [DOI] [PubMed] [Google Scholar]
- KNIGHT R. H., YOUNG L. Biochemical studies of toxic agents. 11. The occurrence of premercapturic acids. Biochem J. 1958 Sep;70(1):111–119. doi: 10.1042/bj0700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY A. The enzymic synthesis of aryl sulphamates. Biochem J. 1960 Jan;74:49–56. doi: 10.1042/bj0740049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisch J., Pagnucco R., Alfes H., Jantos N., Möllmann H. Mass spectra of derivatives of phenylalkylamines. J Pharm Pharmacol. 1968 Feb;20(2):81–86. doi: 10.1111/j.2042-7158.1968.tb09693.x. [DOI] [PubMed] [Google Scholar]
- SJOERDSMA A., von STUDNITZ W. Dopamine-beta-oxidase activity in man, using hydroxyamphetamine as substrate. Br J Pharmacol Chemother. 1963 Apr;20:278–284. doi: 10.1111/j.1476-5381.1963.tb01467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J. N., SMITHIES R. H., WILLIAMS R. T. Studies in detoxication. 59. The metabolism of alkylbenzenes; the biological reduction of ketones derived from alkylbenzenes. Biochem J. 1954 May;57(1):74–76. doi: 10.1042/bj0570074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICKSTROM A., SALVESEN B. The separation and identification of some sympathomimetic amines by paper partition chromatography. J Pharm Pharmacol. 1952 Sep;4(9):631–635. doi: 10.1111/j.2042-7158.1952.tb13193.x. [DOI] [PubMed] [Google Scholar]


