Abstract
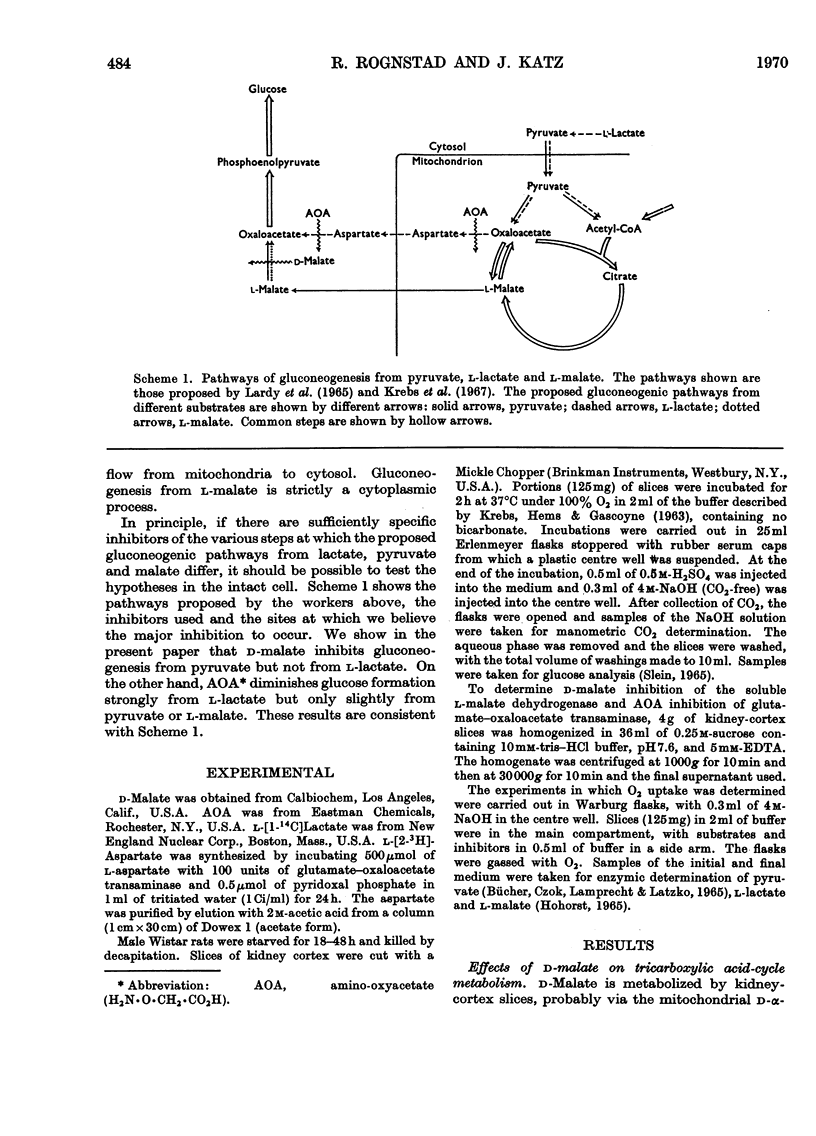
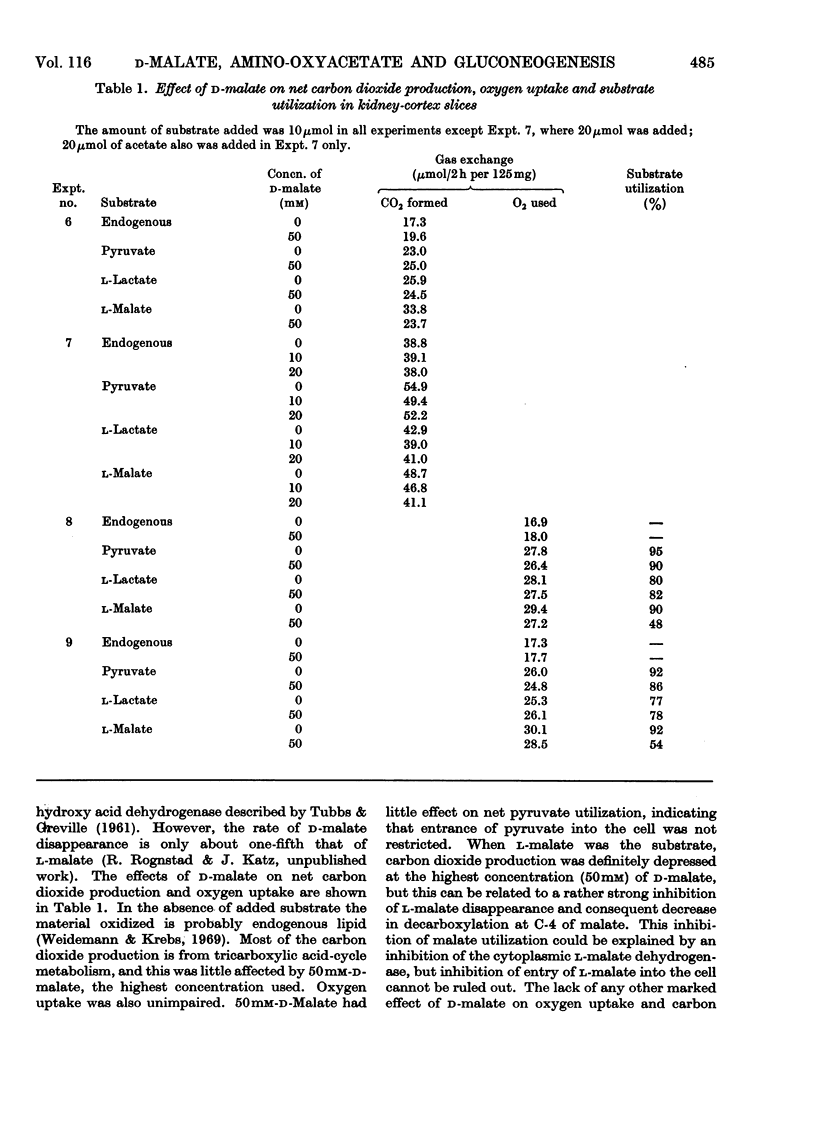
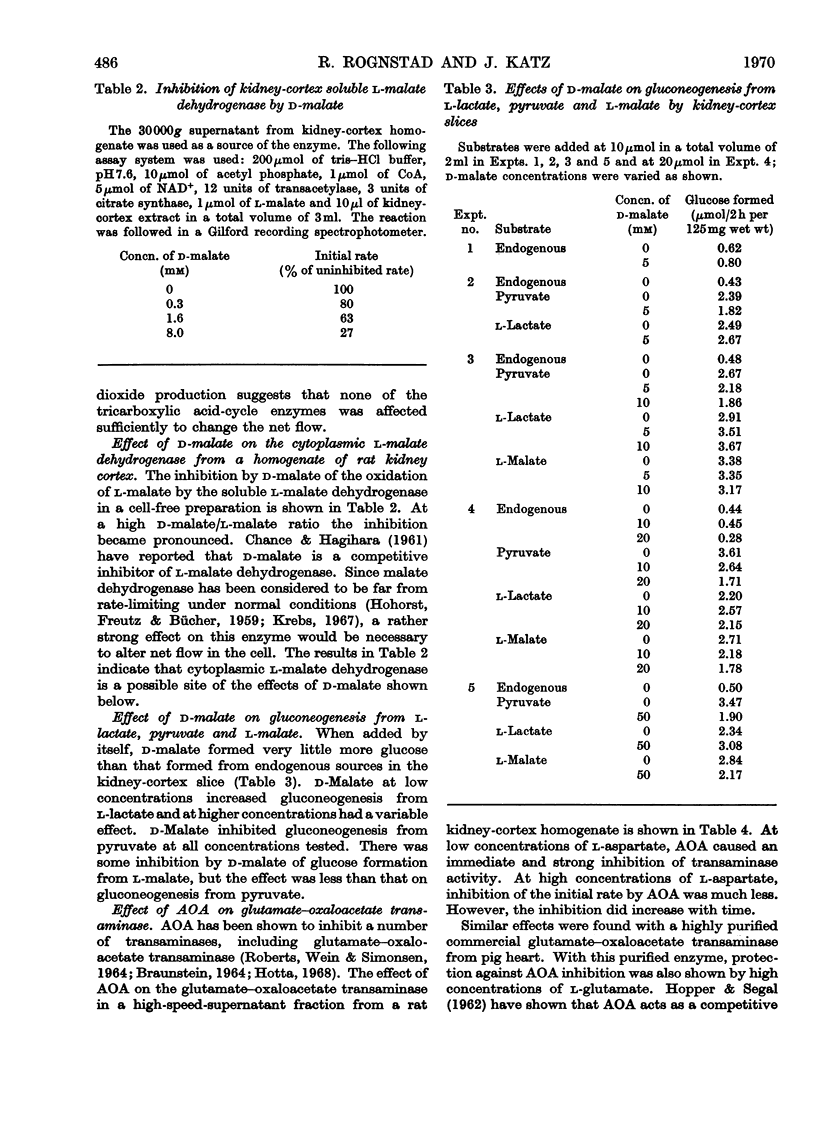
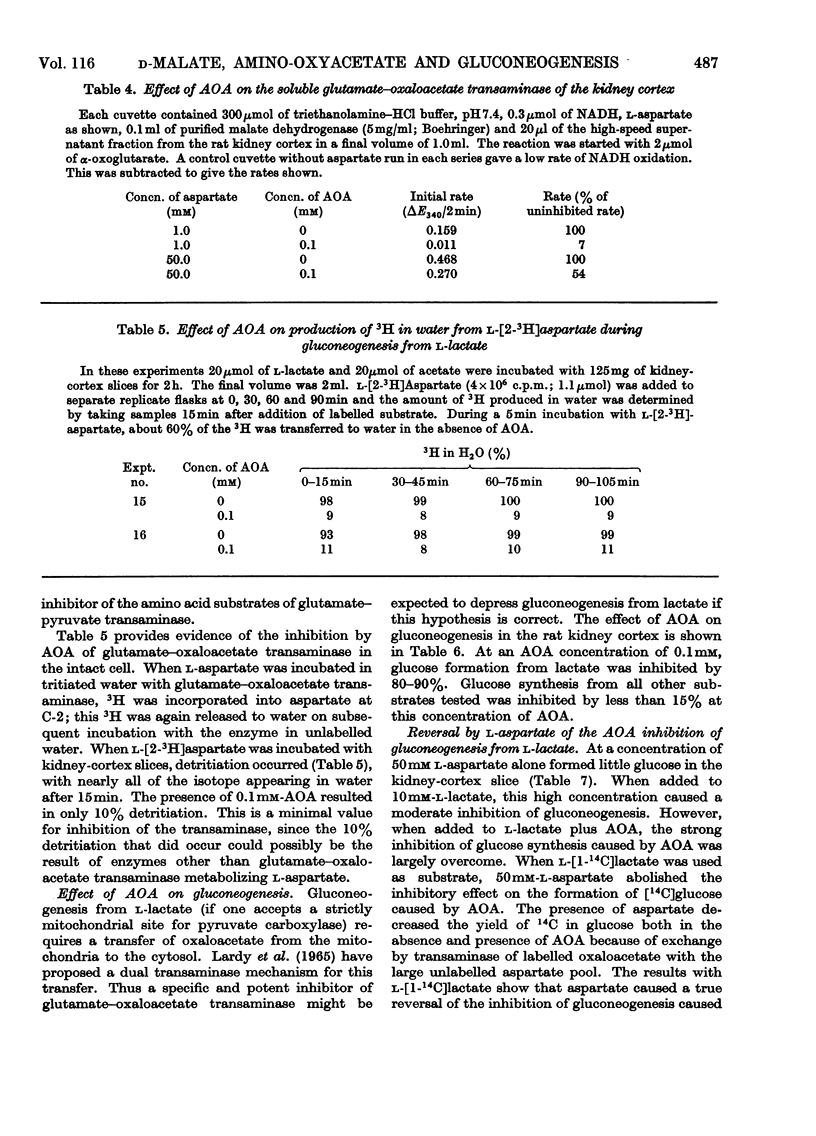
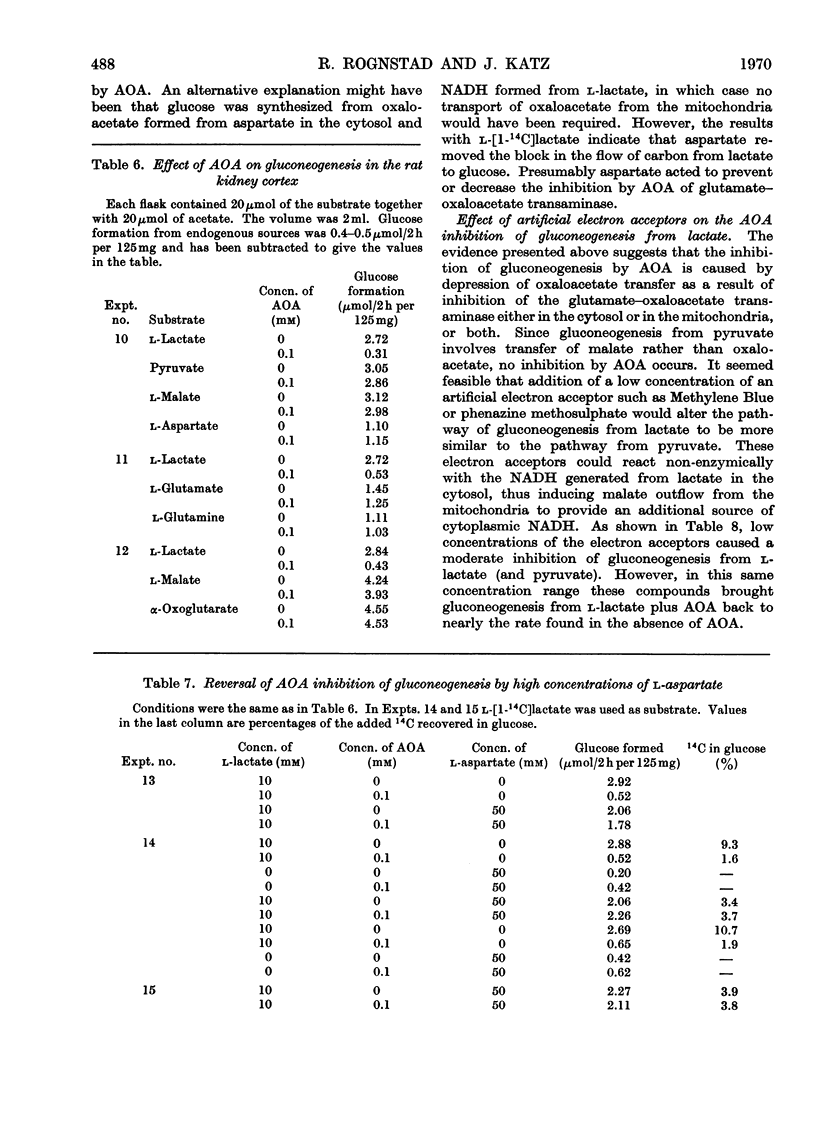
1. Rat kidney-cortex slices incubated with d-malate alone formed very little glucose. d-Malate, however, augmented gluconeogenesis from l-lactate and inhibited gluconeogenesis from pyruvate and l-malate. 2. d-Malate had little effect on the rate of the tricarboxylic acid cycle with or without other substrates added. 3. d-Malate inhibited the activity of the l-malate dehydrogenase in a high-speed-supernatant fraction from kidney cortex. 4. It was concluded that d-malate inhibited either the operation of the cytoplasmic l-malate dehydrogenase or malate outflow from the mitochondria in the intact kidney-cortex cell. This supports the hypothesis of Lardy, Paetkau & Walter (1965) and Krebs, Gascoyne & Notton (1967) on the role of malate as carrier for carbon and reducing equivalents in gluconeogenesis. 5. Gluconeogenesis from l-lactate in kidney-cortex slices was strongly inhibited by a low concentration (0.1mm) of amino-oxyacetate, whereas glucose formation from pyruvate, malate, aspartate and several other compounds was only slightly affected. 6. High concentrations of l-aspartate largely reversed the inhibition of gluconeogenesis from l-lactate caused by amino-oxyacetate. 7. Amino-oxyacetate inhibited strongly the glutamate–oxaloacetate transaminase in the 30000g supernatant fraction of a kidney-cortex homogenate. The presence of l-aspartate decreased the inhibition of the transaminase by amino-oxyacetate. 8. Detritiation of l-[2-3H]aspartate was inhibited by 90% during an incubation of kidney-cortex slices with l-lactate and amino-oxyacetate. 9. Low concentrations (10μm) of artificial electron acceptors such as Methylene Blue and phenazine methosulphate abolished most of the inhibition of gluconeogenesis from l-lactate by amino-oxyacetate. This is interpreted as an activation of net malate outflow from the mitochondria by-passing the inhibited transfer of oxaloacetate. 10. These findings support the concept that transamination to aspartate is involved in the transfer of oxaloacetate from mitochondria to cytosol required in gluconeogenesis from l-lactate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUNSTEIN A. E. BINDING AND REACTIONS OF THE VITAMIN B6 COENZYME IN THE CATALYTIC CENTER OF ASPARTATE TRANSAMINASE. Vitam Horm. 1964;22:451–484. doi: 10.1016/s0083-6729(08)60348-9. [DOI] [PubMed] [Google Scholar]
- Böttger I., Wieland O., Brdiczka D., Pette D. Intracellular localization of pyruvate carboxylase and phosphoenolpyruvate carboxykinase in rat liver. Eur J Biochem. 1969 Mar;8(1):113–119. doi: 10.1111/j.1432-1033.1969.tb00503.x. [DOI] [PubMed] [Google Scholar]
- HOPPER S., SEGAL H. L. Kinetic studies of rat liver glutamicalanine transaminase. J Biol Chem. 1962 Oct;237:3189–3195. [PubMed] [Google Scholar]
- Haslam J. M., Griffiths D. E. Factors affecting the translocation of oxaloacetate and L-malate into rat liver mitochondria. Biochem J. 1968 Oct;109(5):921–928. doi: 10.1042/bj1090921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R. C., Jr The fixation of carbon dioxide by rat liver mitochondria and its relation to gluconeogenesis. J Biol Chem. 1965 Oct;240(10):4103–4106. [PubMed] [Google Scholar]
- Hotta S. S. Oxidative metabolism of isolated brain mitochondria: changes caused by aminooxyacetate. Arch Biochem Biophys. 1968 Sep 20;127(1):132–139. doi: 10.1016/0003-9861(68)90209-9. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R., GASCOYNE T. RENAL GLUCONEOGENESIS. IV. GLUCONEOGENESIS FROM SUBSTRATE COMBINATIONS. Acta Biol Med Ger. 1963;11:607–615. [PubMed] [Google Scholar]
- Krebs H. A., Gascoyne T., Notton B. M. Generation of extramitochondrial reducing power in gluconeogenesis. Biochem J. 1967 Jan;102(1):275–282. doi: 10.1042/bj1020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. The redox state of nicotinamide adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Adv Enzyme Regul. 1967;5:409–434. doi: 10.1016/0065-2571(67)90029-5. [DOI] [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- Lardy H. A., Paetkau V., Walter P. Paths of carbon in gluconeogenesis and lipogenesis: the role of mitochondria in supplying precursors of phosphoenolpyruvate. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1410–1415. doi: 10.1073/pnas.53.6.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlman M. A., Walter P., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. VII. The synthesis of precursors for gluconeogenesis from pyruvate and bicarbonate by rat kidney mitochondria. J Biol Chem. 1967 Oct 25;242(20):4594–4602. [PubMed] [Google Scholar]
- ROBERTS E., WEIN J., SIMONSEN D. G. GAMMA-AMINOBUTYRIC ACID (GABA), VITAMIN B6, AND NEURONAL FUNCTION--A SPECULATIVE SYNTHESIS. Vitam Horm. 1964;22:503–559. [PubMed] [Google Scholar]
- Schmidt K., Katz J. Metabolism of pyruvate and L-lactate by rat adipose tissue. J Biol Chem. 1969 Apr 25;244(8):2125–2131. [PubMed] [Google Scholar]
- Seubert W., Huth W. On the mechanism of gluconeogenesis and its regulation. II. The mechanism of gluconeogenesis from pyruvate and fumarate. Biochem Z. 1965 Nov 15;343(2):176–191. [PubMed] [Google Scholar]
- Shrago E., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. II. Conversion of precursors to phosphoenolpyruvate in liver cytosol. J Biol Chem. 1966 Feb 10;241(3):663–668. [PubMed] [Google Scholar]
- TUBBS P. K., GREVILLE G. D. The oxidation of D-alpha-hydroxy acids in animal tissues. Biochem J. 1961 Oct;81:104–114. doi: 10.1042/bj0810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]


