Abstract
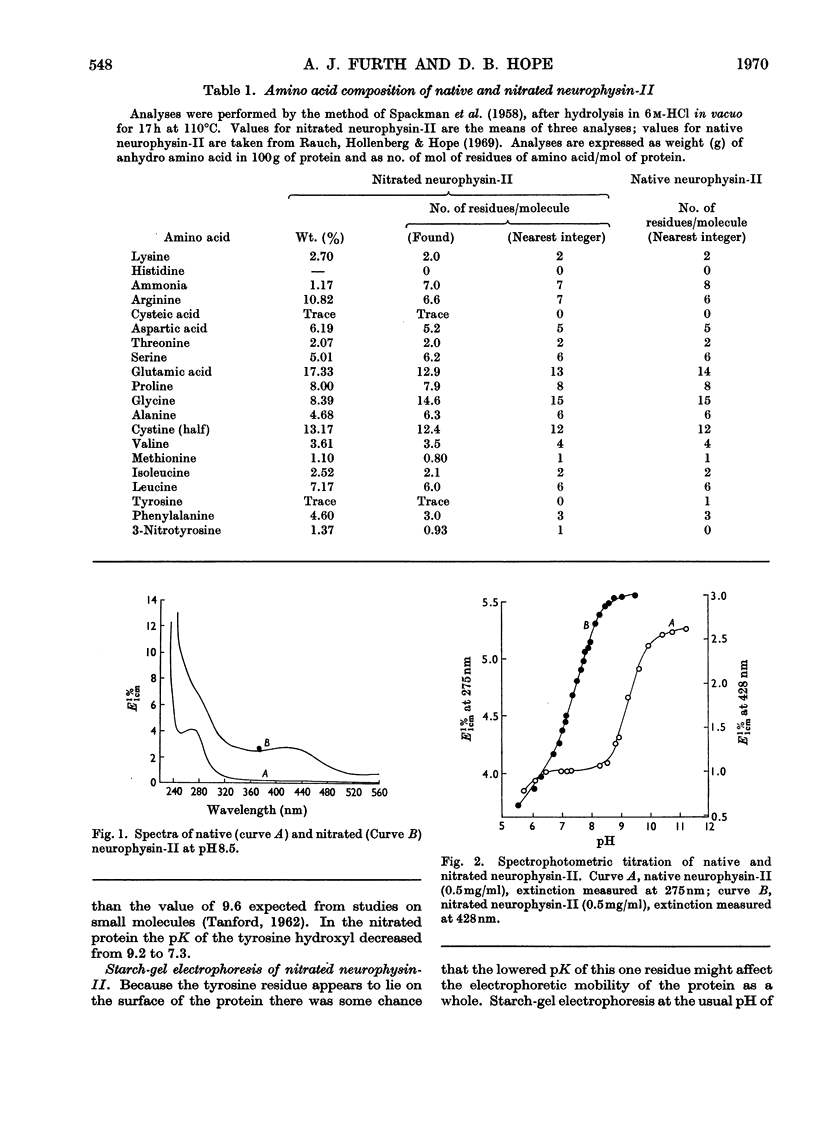
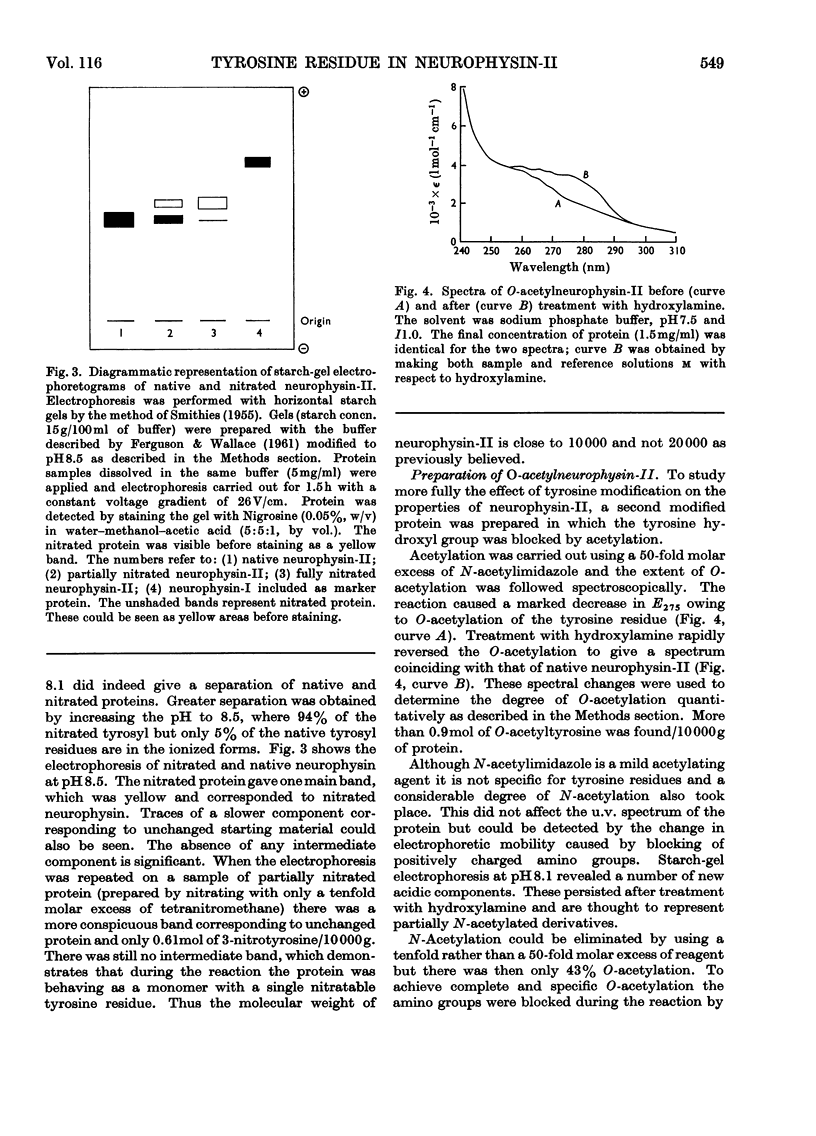
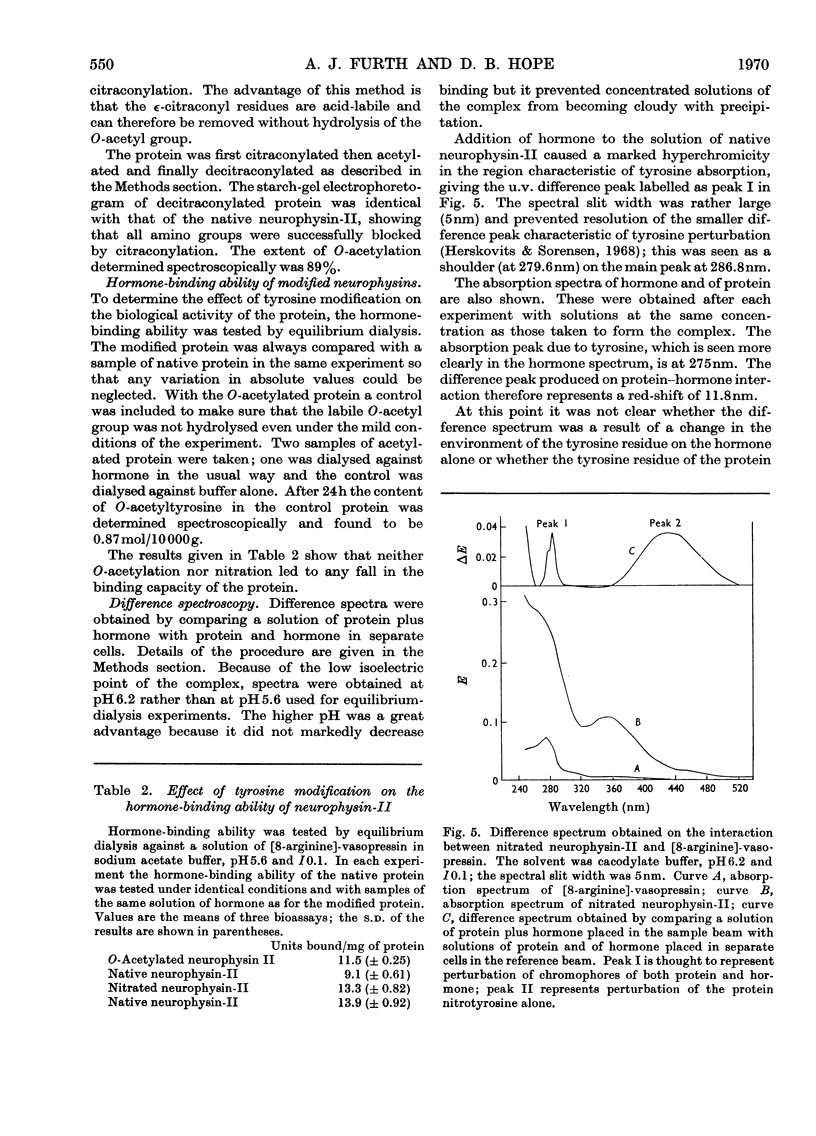
1. Bovine neurophysin-II contains 1mol of tyrosine residue/10000g of protein. This residue could be readily nitrated with tetranitromethane. On hydrolysis and amino acid analysis 1mol of 3-nitrotyrosine was found/10000g of protein. Starchgel electrophoresis at pH8.5 showed that nitration had converted the native protein into a single, more acidic species. The increase in acidity was consistent with the observed fall in pK of the tyrosine hydroxyl from 9.2 in native neurophysin to 7.3 in the nitrated protein. Further, the absence of any intermediate species, even under conditions of minimum substitution, confirmed that the molecular weight of the monomer is 10000. 2. O-Acetylation of the tyrosine residue was carried out with N-acetylimidazole, in conjunction with the reversible blocking of amino groups by citraconylation. The degree of O-acetylation, determined spectroscopically, was 0.9mol of O-acetyltyrosine/10000g of protein. 3. The hormone-binding ability of modified protein was tested by equilibrium dialysis and was found to be unchanged by either nitration or O-acetylation of the tyrosine residue. 4. Interaction of neurophysin-II and [8-arginine]-vasopressin gave rise to a characteristic difference spectrum with a peak at 286.8nm and shoulder at 279.6nm. Part of this hyperchromicity is thought to result from entry of the tyrosine residue at position 2 in the hormone into the hydrophobic environment of the binding site. With nitrated neurophysin-II a second peak appeared at 436nm, showing that the tyrosine of the protein is also perturbed. The very large red shift (84nm) in this region suggests that the 3-nitrotyrosyl residue not only enters a more hydrophobic environment on protein–hormone interaction, but is caused to ionize more fully by the approach of some positively charged group.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., CHAUVET J., OLIVRY G. Sur l'existence éventuelle d'une hormone unique neurohypophysaire. I. Relations entre l'ocytocine, la vasopressine et la protéine de Van Dyke extraites de la neurohypophyse du boeuf. Biochim Biophys Acta. 1956 Dec;22(3):421–427. doi: 10.1016/0006-3002(56)90050-6. [DOI] [PubMed] [Google Scholar]
- BANGHAM D. R., MUSSETT M. V. Third international standard for posterior pituitary; re-named third international standard for oxytocic, vasopressor and antidiuretic substances in 1956. Bull World Health Organ. 1958;19(2):325–340. [PMC free article] [PubMed] [Google Scholar]
- BURR M., KOSHLAND D. E., Jr USE OF "REPORTER GROUPS" IN STRUCTURE-FUNCTION STUDIES OF PROTEINS. Proc Natl Acad Sci U S A. 1964 Oct;52:1017–1024. doi: 10.1073/pnas.52.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow E., Abrash L. The binding of oxytocin and oxytocin analogues by purified bovine neurophysins. Proc Natl Acad Sci U S A. 1966 Aug;56(2):640–646. doi: 10.1073/pnas.56.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Fuchs S., Anfinsen C. B. The tyrosyl residues at the active site of staphylococcal nuclease. Modifications by tetranitromethane. J Biol Chem. 1968 Sep 25;243(18):4787–4798. [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Hope D. B. The isolation of purified neurosecretory granules from bovine pituitary posterior lobes. Comparison of granule protein constituents with those of neurophysin. Biochem J. 1967 Sep;104(3):1082–1088. doi: 10.1042/bj1041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon H. B., Perham R. N. Reversible blocking of amino groups with citraconic anhydride. Biochem J. 1968 Sep;109(2):312–314. doi: 10.1042/bj1090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON K. A., WALLACE A. L. Starch-gel electrophoresis of anterior pituitary hormones. Nature. 1961 May 13;190:629–630. doi: 10.1038/190629a0. [DOI] [PubMed] [Google Scholar]
- Frankland B. T., Hollenberg M. D., Hope D. B., Schacter B. A. Dissociation of oxytocin and vasopressin from their carrier protein by chromatography on sephadex G-25. Br J Pharmacol Chemother. 1966 Feb;26(2):502–510. doi: 10.1111/j.1476-5381.1966.tb01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits T. T., Sorensen M. Studies of the location of tyrosyl and tryptophyl residues in proteins. I. Solvent perturbation data of model compounds. Biochemistry. 1968 Jul;7(7):2523–2532. doi: 10.1021/bi00847a012. [DOI] [PubMed] [Google Scholar]
- Hettinger T. P., Kurylo-Borowska Z., Craig L. C. Edeine. 3. The composition of the antibiotic peptide edeine B. Biochemistry. 1968 Dec;7(12):4153–4160. doi: 10.1021/bi00852a002. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. The composition of crystalline complexes of neurophysin-M with [8-arginine]-vasopressin and oxytocin. Biochem J. 1967 Dec;105(3):921–926. doi: 10.1042/bj1050921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. The isolation of the native hormone-binding proteins from bovine pituitary posterior lobes. Crystallization of neurophysin-I and-II as complexes with [8-arginine]-vasopressin. Biochem J. 1968 Jan;106(2):557–564. doi: 10.1042/bj1060557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meloun B., Fric I., Sorm F. Nitration of tyrosine residues in the pancreatic trypsin inhibitor with tetranitromethane. Eur J Biochem. 1968 Mar;4(1):112–117. doi: 10.1111/j.1432-1033.1968.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Lindskog S. Hydrogen ion equilibria and the chemical modification of lysine and tyrosine residues in bovine carbonic anhydrase B. Eur J Biochem. 1967 Oct;2(3):309–317. doi: 10.1111/j.1432-1033.1967.tb00140.x. [DOI] [PubMed] [Google Scholar]
- RICHARDS F. M., LOGUE A. D. Changes in absorption spectra in the ribonuclease-S system. J Biol Chem. 1962 Dec;237:3693–3697. [PubMed] [Google Scholar]
- Rauch R., Hollenberg M. D., Hope D. B. Isolation of a third bovine neurophysin. Biochem J. 1969 Nov;115(3):473–479. doi: 10.1042/bj1150473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYER W. H. Neurophypophysial hormones. Pharmacol Rev. 1961 Jun;13:225–277. [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- Wyckoff H. W., Hardman K. D., Allewell N. M., Inagami T., Johnson L. N., Richards F. M. The structure of ribonuclease-S at 3.5 A resolution. J Biol Chem. 1967 Sep 10;242(17):3984–3988. [PubMed] [Google Scholar]


