Abstract
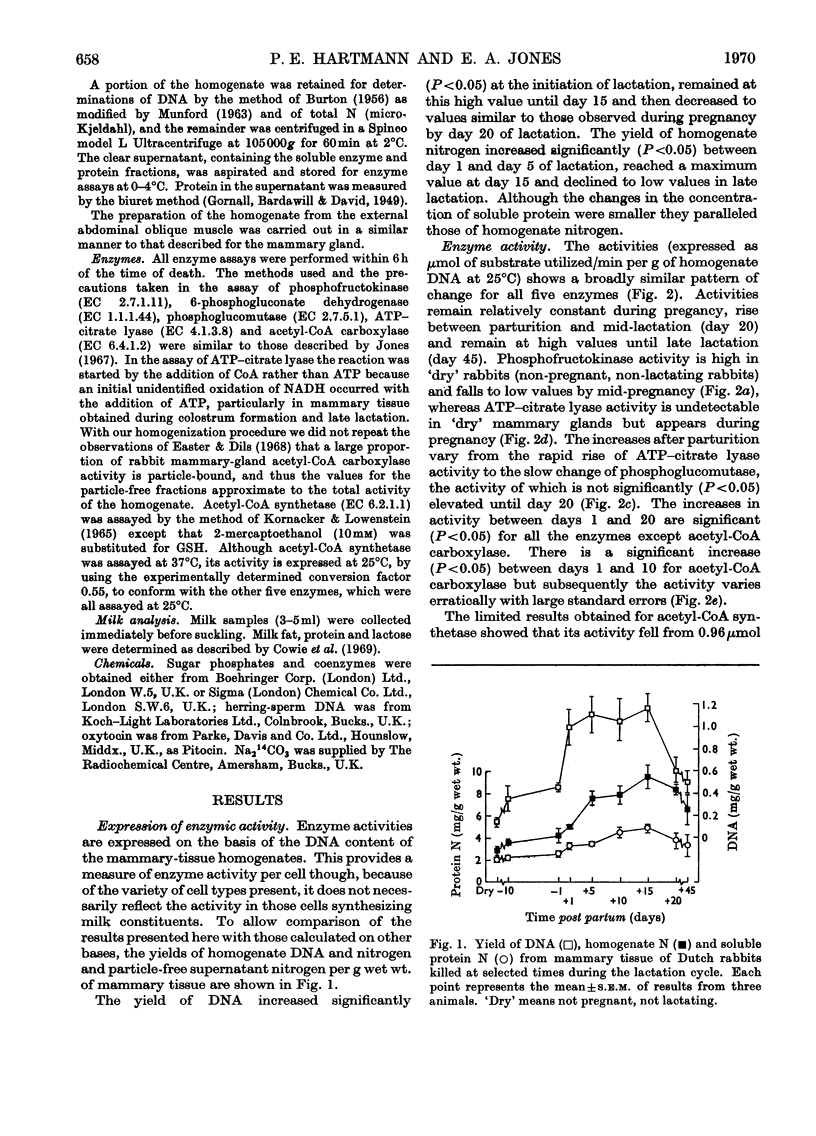
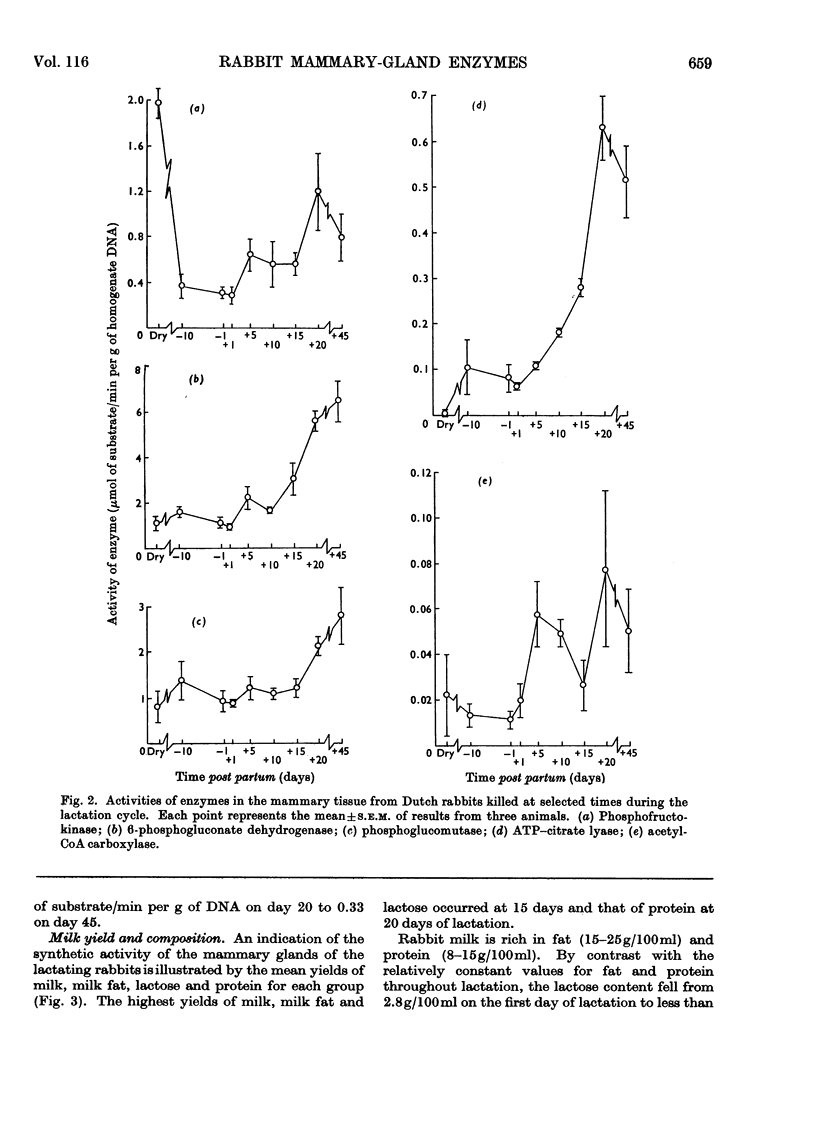
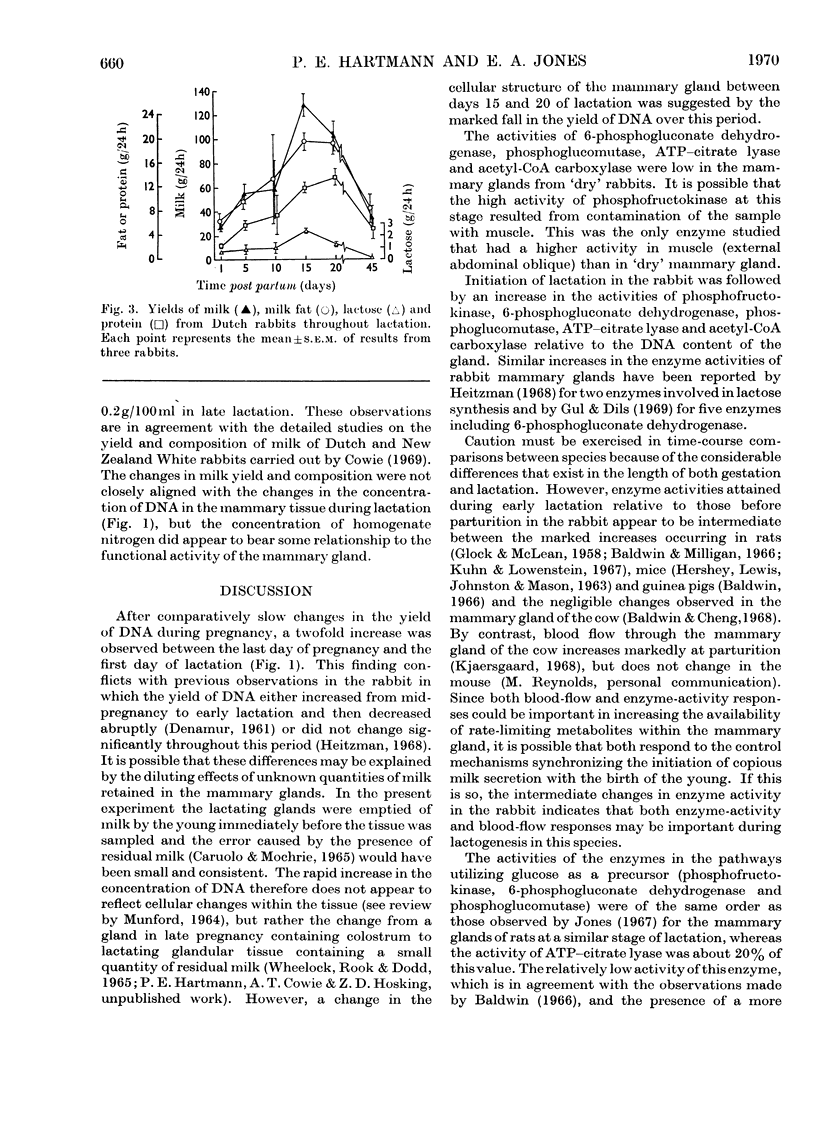
1. The enzymes phosphofructokinase (EC 2.7.1.11), 6-phosphogluconate dehydrogenase (EC 1.1.1.44), phosphoglucomutase (EC 2.7.5.1), ATP–citrate lyase (EC 4.1.3.8), acetyl-CoA carboxylase (EC 6.4.1.2) and acetyl-CoA synthetase (EC 6.2.1.1) were assayed in rabbit mammary glands at various stages of the pregnancy–lactation cycle. 2. The activities of all enzymes were low during pregnancy and, with the exception of phosphofructokinase, in non-pregnant animals. Two- to ten-fold increases in enzyme activities occurred over the first 20 days of lactation. Although milk yield was considerably decreased, the enzyme activities remained elevated in late lactation (45 days after parturition). 3. These findings are discussed in relation to mammary-gland metabolism and compared with similar observations previously made on ruminants and other small mammals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. L. Enzymatic activities in mammary glands of several species. J Dairy Sci. 1966 Dec;49(12):1533–1542. doi: 10.3168/jds.S0022-0302(66)88132-8. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L., Milligan L. P. Enzymatic changes associated with the initiation and maintenance of lactation in the rat. J Biol Chem. 1966 May 10;241(9):2058–2066. [PubMed] [Google Scholar]
- Cowie A. T., Hartmann P. E., Turvey A. The maintenance of lactation in the rabbit after hypophysectomy. J Endocrinol. 1969 Apr;43(4):651–662. doi: 10.1677/joe.0.0430651. [DOI] [PubMed] [Google Scholar]
- Cowie A. T. Variations in the yield and composition of the milk during lactation in the rabbit and the galactopoietic effect of prolactin. J Endocrinol. 1969 Jul;44(3):437–450. doi: 10.1677/joe.0.0440437. [DOI] [PubMed] [Google Scholar]
- Easter D. J., Dils R. Fatty acid biosynthesis. IV. Properties of acetyl-CoA carboxylase in lactating-rabbit mammary gland. Biochim Biophys Acta. 1968 Jul 1;152(4):653–668. doi: 10.1016/0005-2760(68)90112-4. [DOI] [PubMed] [Google Scholar]
- Folley S. J., Watson S. C. A high-speed tissue homogenizer. Biochem J. 1948;42(2):204–206. doi: 10.1042/bj0420204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul B., Dils R. Enzymic changes in rabbit and rat mammary gland during the lactation cycle. Biochem J. 1969 Apr;112(3):293–301. doi: 10.1042/bj1120293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick D. C. The fate of acetyl groups derived from glucose in the isolated perfused goat udder. Biochem J. 1966 Apr;99(1):228–231. doi: 10.1042/bj0990228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzman R. J. Enzymes of lactose biosynthesis in normal and hormonally stimulated rabbit mammary glands. J Endocrinol. 1968 Jan;40(1):81–84. doi: 10.1677/joe.0.0400081. [DOI] [PubMed] [Google Scholar]
- Jones E. A. Changes in the enzyme pattern of the mammary gland of the lactating rat after hypophysectomy and weaning. Biochem J. 1967 May;103(2):420–427. doi: 10.1042/bj1030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaersgaard P. Mammary blood flow ante and post partum in cows. Acta Vet Scand. 1968;9(2):180–181. doi: 10.1186/BF03547884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., Lowenstein J. M. Lactogenesis in the rat. Changes in metabolic parameters at parturition. Biochem J. 1967 Dec;105(3):995–1002. doi: 10.1042/bj1050995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNFORD R. E. CHANGES IN THE MAMMARY GLANDS OF RATS AND MICE DURING PREGNANCY, LACTATION AND INVOLUTION. 2. LEVELS OF DEOXYRIBONUCLEIC ACID, AND ALKALINE AND ACID PHOSPHATASES. J Endocrinol. 1963 Dec;28:17–34. doi: 10.1677/joe.0.0280017. [DOI] [PubMed] [Google Scholar]
- McLEAN P. Carbohydrate metabolism of mammary tissue. I. Pathways of glucose catabolism in the mammary gland. Biochim Biophys Acta. 1958 Nov;30(2):303–315. doi: 10.1016/0006-3002(58)90055-6. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. What regulates lactose content in milk? Nature. 1969 Mar 8;221(5184):912–914. doi: 10.1038/221912a0. [DOI] [PubMed] [Google Scholar]
- Smith S., Watts R., Dils R. Quantitative gas-liquid chromatographic analysis of rodent milk triglycerides. J Lipid Res. 1968 Jan;9(1):52–57. [PubMed] [Google Scholar]


