Abstract
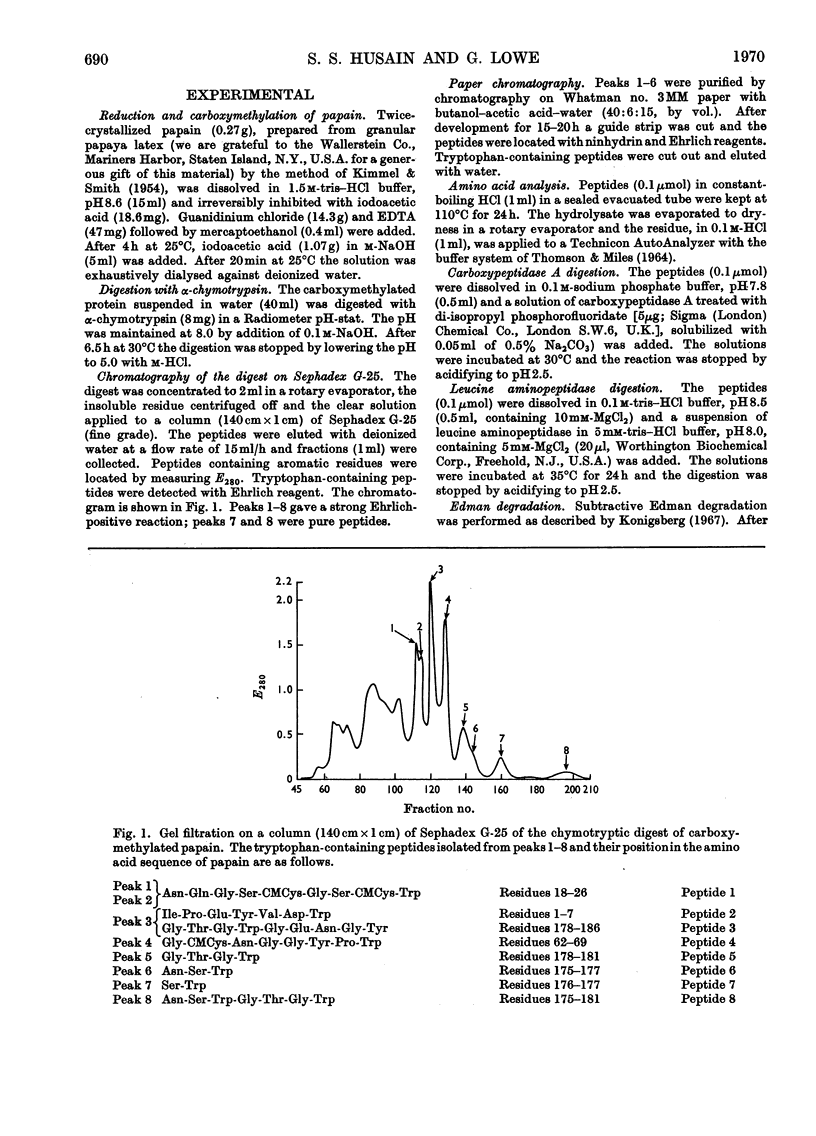
The tryptophan-containing peptides were isolated from the chymotryptic digest of S-carboxymethylated papain. Residue 175, which is strongly hydrogen-bonded to the active-site histidine residue in the tertiary structure of papain, is asparagine, confirming the work of Kimmel, Rogers & Smith (1965). Its function is probably to maintain the orientation and tautomeric state of the imidazole ring of histidine-159. The amino acid sequence predicted from the electron-density map of papain for residues 64–68 was confirmed, but residue 64 is asparagine, not aspartic acid. This residue, which is about 10 Å from the thiol group of the active-site cysteine-25, cannot therefore be a site of electrostatic attraction for substrates of basic amino acids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alden R. A., Wright C. S., Kraut J. A hydrogen-bond network at the active site of subtilisin BPN'. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):119–124. doi: 10.1098/rstb.1970.0014. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Sluyterman L. A., Wolthers B. G. IV. Cysteine proteinases. The structure of the papain molecule. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):231–236. doi: 10.1098/rstb.1970.0022. [DOI] [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Swen H. M., Wolthers B. G. Structure of papain. Nature. 1968 Jun 8;218(5145):929–932. doi: 10.1038/218929a0. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. Completion of the amino acid sequence of papain. Biochem J. 1969 Sep;114(2):279–288. doi: 10.1042/bj1140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. Evidence for histidine in the active site of papain. Biochem J. 1968 Aug;108(5):855–859. doi: 10.1042/bj1080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. The location of the active-site histidine residue in the primary sequence of papain. Biochem J. 1968 Aug;108(5):861–866. doi: 10.1042/bj1080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMMEL J. R., ROGERS H. J., SMITH E. L. TRYPTIC DIGEST OF PAPAIN. II. ISOLATION AND SEQUENCES OF PEPTIDES FROM THE CARBOXYMETHYLATED PROTEIN. J Biol Chem. 1965 Jan;240:266–280. [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- LIGHT A., FRATER R., KIMMEL J. R., SMITH E. L. CURRENT STATUS OF THE STRUCTURE OF PAPAIN: THE LINEAR SEQUENCE, ACTIVE SULFHYDRYL GROUP, AND THE DISULFIDE BRIDGES. Proc Natl Acad Sci U S A. 1964 Nov;52:1276–1283. doi: 10.1073/pnas.52.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. W., Sigler P. B., Henderson R., Blow D. M. Three-dimensional structure of tosyl-alpha-chymotrypsin. Nature. 1967 May 13;214(5089):652–656. doi: 10.1038/214652a0. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Sigler P. B., Blow D. M., Matthews B. W., Henderson R. Structure of crystalline -chymotrypsin. II. A preliminary report including a hypothesis for the activation mechanism. J Mol Biol. 1968 Jul 14;35(1):143–164. doi: 10.1016/s0022-2836(68)80043-9. [DOI] [PubMed] [Google Scholar]
- THOMSON A. R., MILES B. J. ION-EXCHANGE CHROMATOGRAPHY OF AMINO-ACIDS: IMPROVEMENTS IN THE SINGLE COLUMN SYSTEM. Nature. 1964 Aug 1;203:483–484. doi: 10.1038/203483a0. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Parker L. Pretransition-state protonation and the rate of chymotrypsin catalysis. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2451–2454. doi: 10.1073/pnas.58.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]


