Abstract
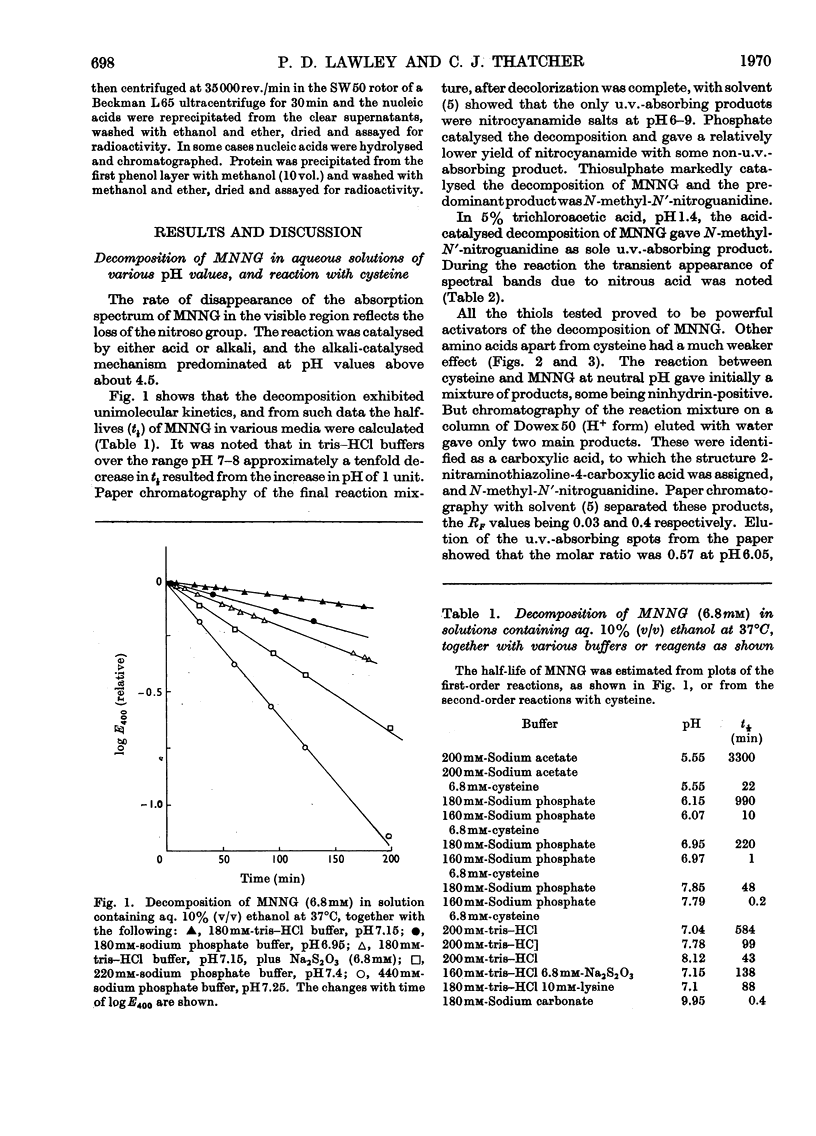
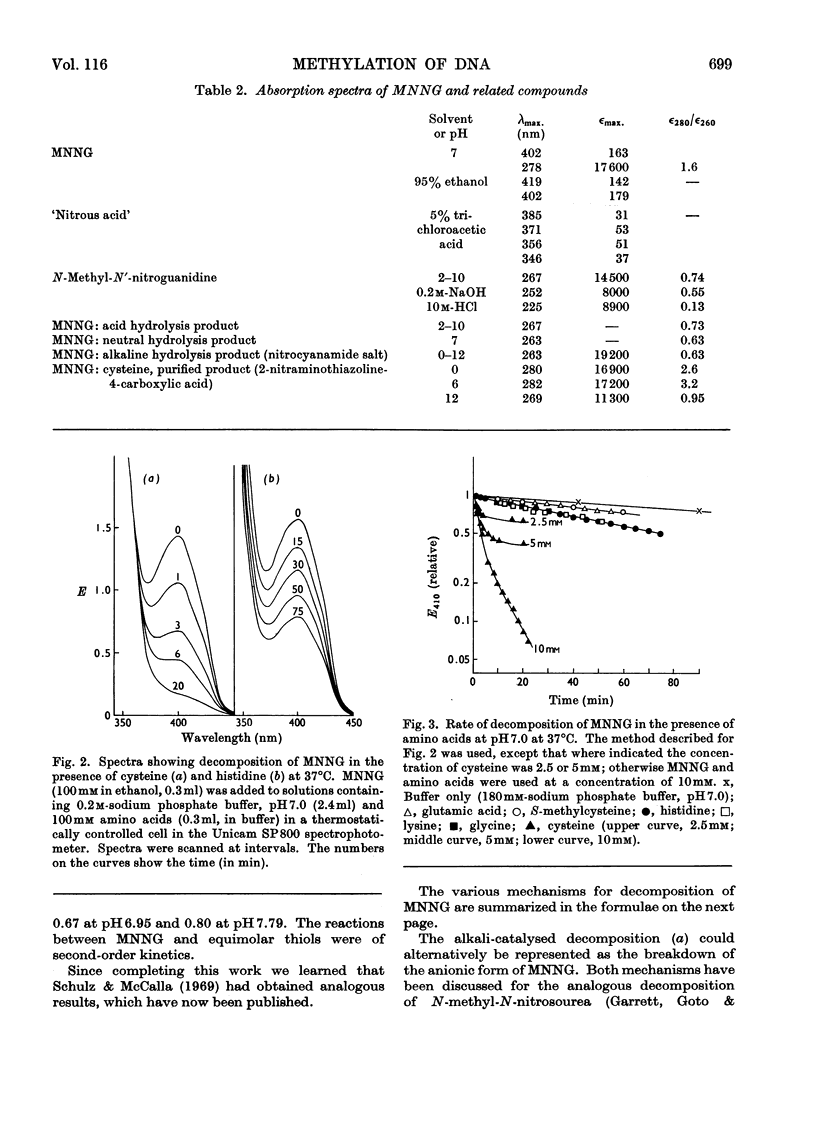
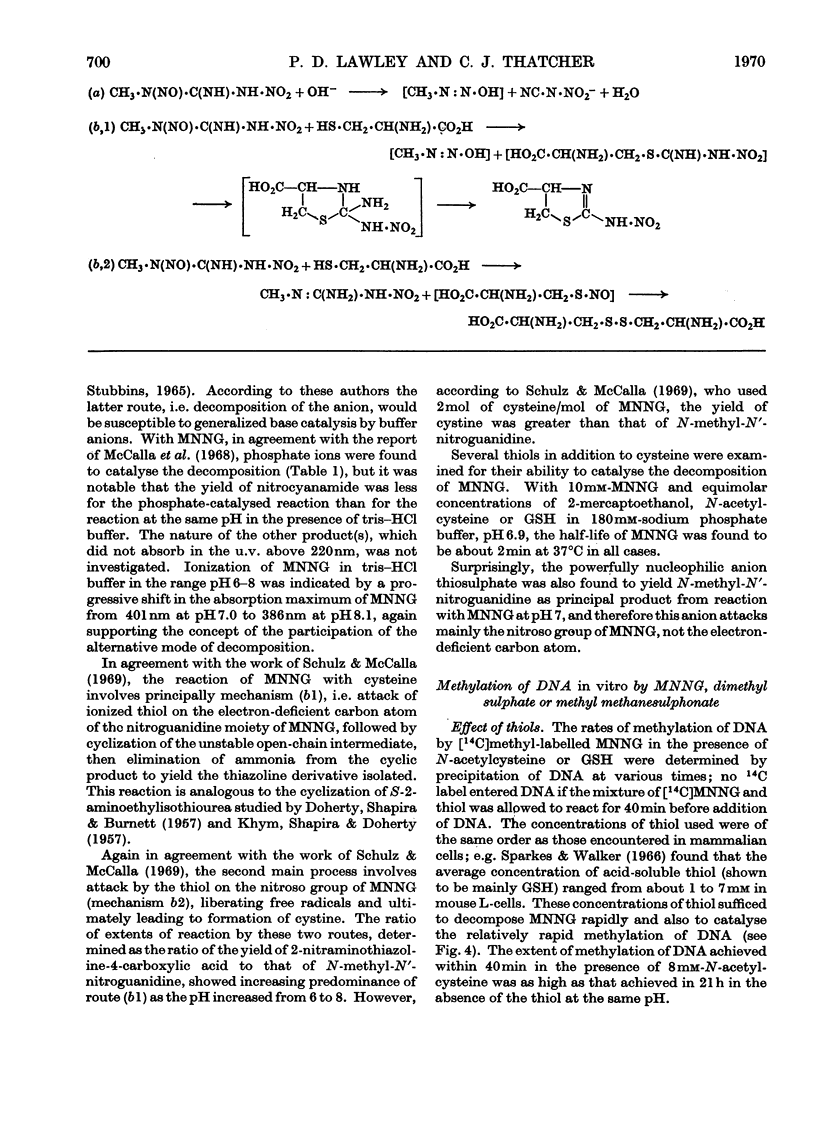
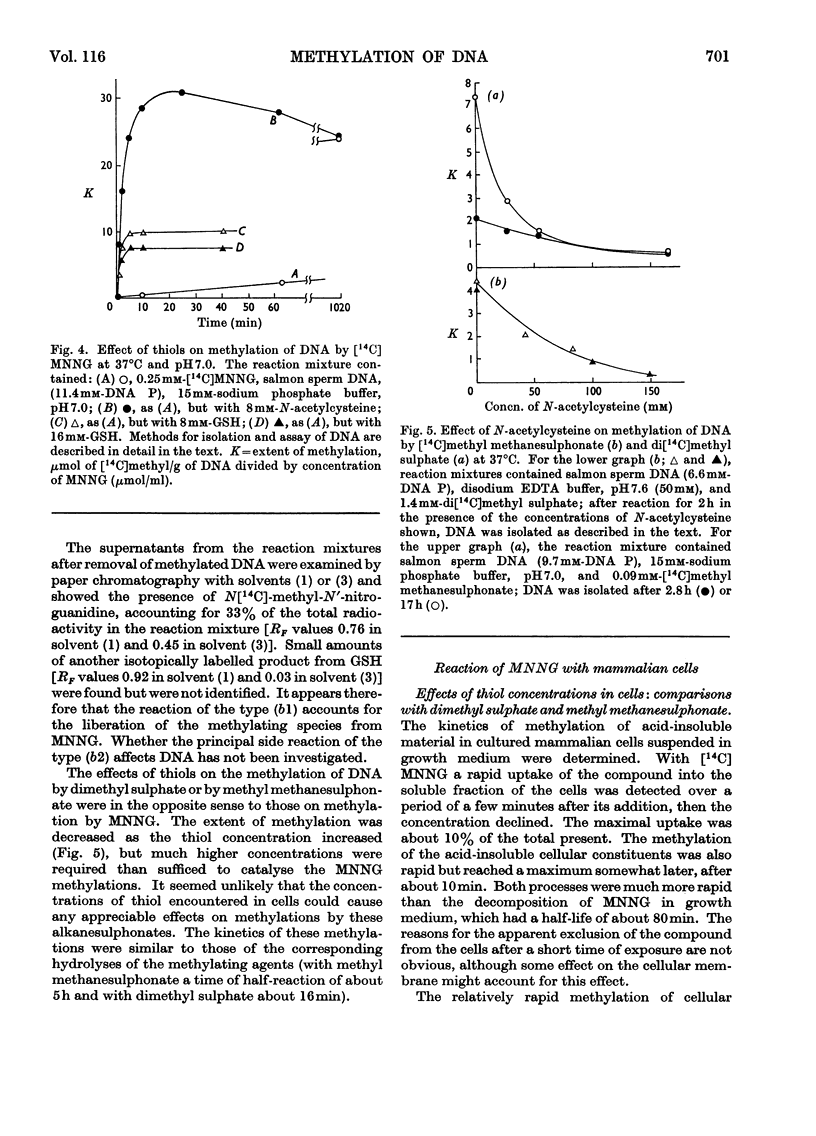
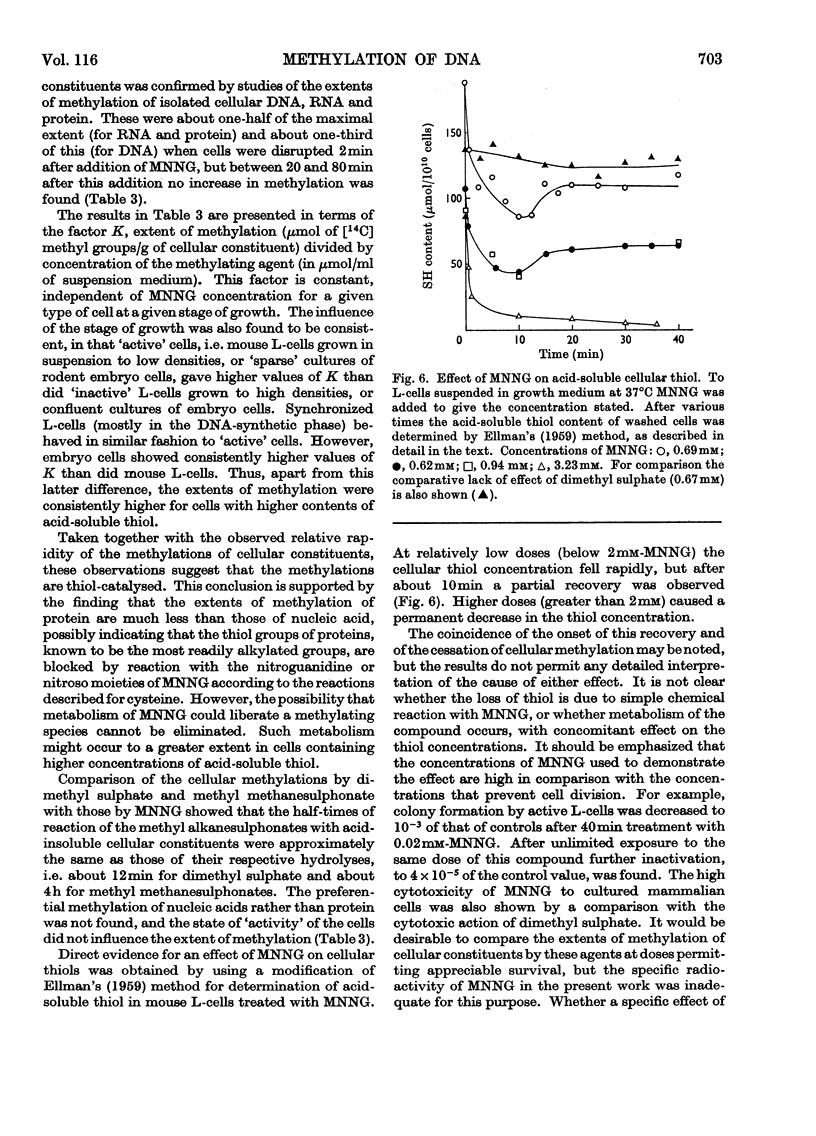
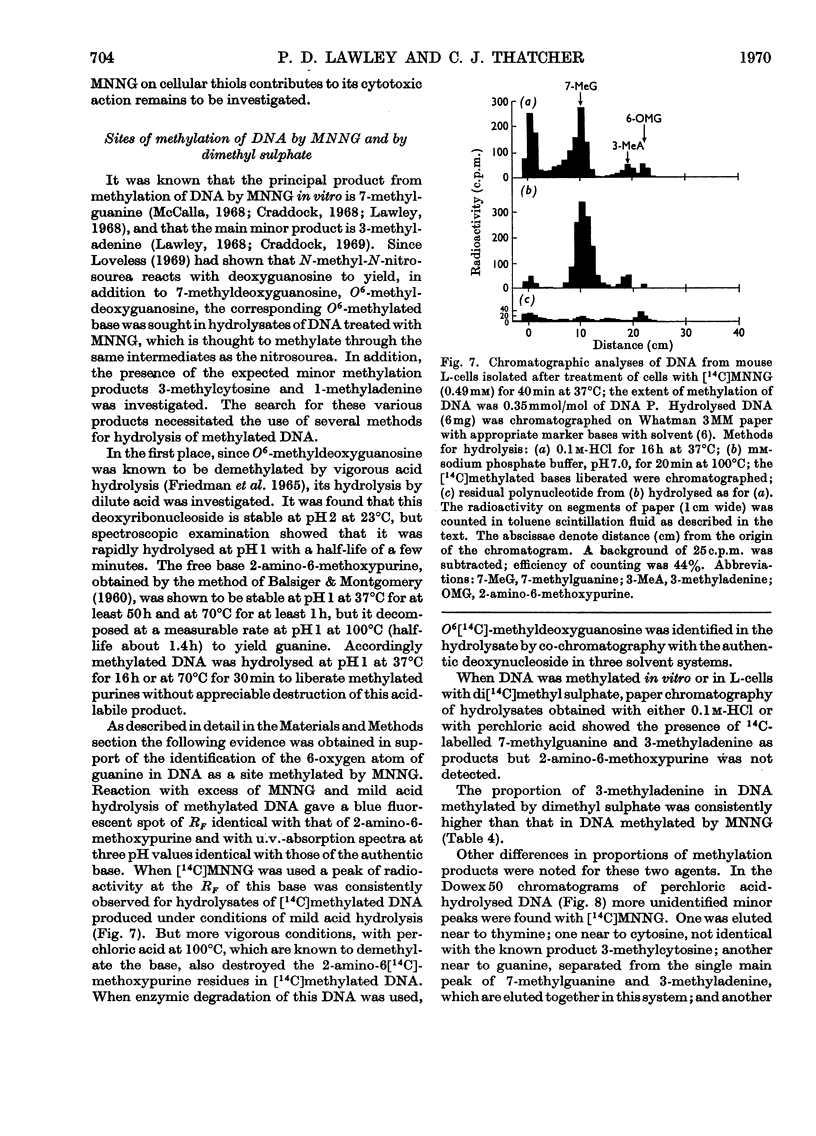
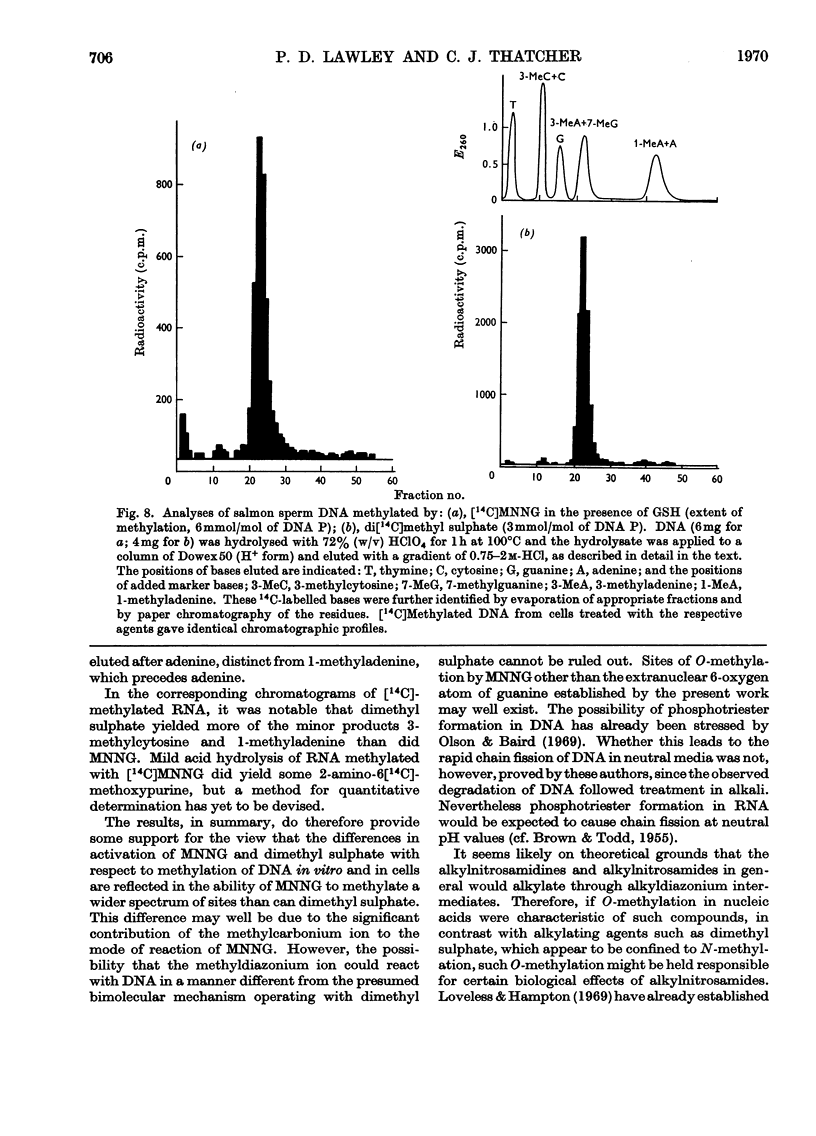
1. In neutral aqueous solution N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) yields salts of nitrocyanamide as u.v.-absorbing products. With cysteine, as found independently by Schulz & McCalla (1969), the principal product is 2-nitràminothiazoline-4-carboxylic acid. Both these reactions liberate the methylating species; thiols enhance the rate markedly at neutral pH values. An alternative reaction with thiols gives cystine, presumably via the unstable S-nitrosocysteine. 2. Thiols (glutathione or N-acetylcysteine) in vitro at about the concentration found in mammalian cells enhance the rate of methylation of DNA markedly over that in neutral solution. 3. Treatment of cultured mammalian cells with MNNG results in rapid methylation of nucleic acids, the extent being greater the higher the thiol content of the cells. Rodent embryo cells are more extensively methylated than mouse L-cells of the same thiol content. Cellular thiol concentrations are decreased by MNNG. Proteins are less methylated by MNNG than are nucleic acids. 4. Methylation of cells by dimethyl sulphate does not depend on cellular thiol content and protein is not less methylated than nucleic acids. Methylation by MNNG may therefore be thiol-stimulated in cells. 5. Both in vitro and in cells about 7% of the methylation of DNA by MNNG occurs at the 6-oxygen atom of guanine. The major products 7-methylguanine and 3-methyladenine are given by both MNNG and dimethyl sulphate, but dimethyl sulphate does not yield O6-methylguanine. Possible reaction mechanisms to account for this difference between these methylating agents and its possible significance as a determinant of their biological effects are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craddock V. M. Study of the methylation and lack of deamination of deoxyribonucleic acid by N-methyl-N'-nitro-N-nitrosoguanidine. Biochem J. 1969 Mar;111(5):615–620. doi: 10.1042/bj1110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock V. M. The reaction of N-methyl-N'-nitro-N-nitrosoguanidine with deoxyribonucleic acid. Biochem J. 1968 Feb;106(4):921–922. doi: 10.1042/bj1060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE E. On the molecular explanation of spontaneous and induced mutations. Brookhaven Symp Biol. 1959 Nov;NO:63–75. [PubMed] [Google Scholar]
- Friedman O. M., Mahapatra G. N., Dash B., Stevenson R. Studies on the action of diazomethane on deoxyribonucleic acid. The action of diazomethane on deoxyribonucleosides. Biochim Biophys Acta. 1965 Jun 8;103(2):286–297. doi: 10.1016/0005-2787(65)90168-1. [DOI] [PubMed] [Google Scholar]
- GARRETT E. R., GOTO S., STUBBINS J. F. KINETICS OF SOLVOLYSES OF VARIOUS N-ALKYL-N-NITROSOUREAS IN NEUTRAL AND ALKALINE SOLUTIONS. J Pharm Sci. 1965 Jan;54:119–123. doi: 10.1002/jps.2600540127. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of deoxyribonucleic acids; evidence on the nature of bonds between deoxyribonucleic acid and protein. Biochem J. 1957 Jul;66(3):495–504. doi: 10.1042/bj0660495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. Acidic dissociation of 7:9-dialkylguanines and its possible relation to mutagenic properties of alkylating agents. Nature. 1961 Dec 16;192:1081–1082. doi: 10.1038/1921081b0. [DOI] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVELESS A. The influence of radiomimetic substances on deoxyribonucleic acid synthesis and function studied in Escherichia coli/phage systems. III. Proc R Soc Lond B Biol Sci. 1959 Sep 1;150:497–508. doi: 10.1098/rspb.1959.0038. [DOI] [PubMed] [Google Scholar]
- Lawley P. D. Methylation of DNA by N-methyl-N-nitrosourethane and N-methyl-N-nitroso-N'-nitroguanidine. Nature. 1968 May 11;218(5141):580–581. doi: 10.1038/218580a0. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- MIZRAHI I. J., EMMELOT P. ON THE MODE OF ACTION BY WHICH THE CARCINOGEN DIMETHYLNITROSAMINE INHIBITS PROTEIN SYNTHESIS IN THE LIVER. Biochim Biophys Acta. 1964 Oct 16;91:362–364. doi: 10.1016/0926-6550(64)90271-3. [DOI] [PubMed] [Google Scholar]
- McCalla D. R. Reaction of N-methyl-N'-nitro-N-nitroguanidine and N-methyl-N-nitroso-p-toluenesulfonamide with DNA in vitro. Biochim Biophys Acta. 1968 Jan 29;155(1):114–120. doi: 10.1016/0005-2787(68)90341-9. [DOI] [PubMed] [Google Scholar]
- McCalla D. R., Reuvers A., Kitai R. Inactivation of biologically active N-methyl-N-nitroso compounds in aqueous solution: effect of various conditions of pH and illumination. Can J Biochem. 1968 Aug;46(8):807–811. doi: 10.1139/o68-122. [DOI] [PubMed] [Google Scholar]
- NAGATA C., IMAMURA A., SAITO H., FUKUI K. Changes of pi-electron distribution of deoxyribonucleic acid after alkylation and their possible relation to the biological effect. Gan. 1963 Mar;54:109–117. [PubMed] [Google Scholar]
- Olson A. O., Baird K. M. Single-strand breaks in Escherichia coli DNA caused by treatment with nitrosoguanidine. Biochim Biophys Acta. 1969 Apr 22;179(2):513–514. doi: 10.1016/0005-2787(69)90063-x. [DOI] [PubMed] [Google Scholar]
- SHEININ R. Procedures for the purification of polyoma T virus. Virology. 1962 Jul;17:426–440. doi: 10.1016/0042-6822(62)90138-1. [DOI] [PubMed] [Google Scholar]
- Schoental R. Instability of some of the products formed by the action of N-methyl-N-nitrosourethane on cysteine in vitro. Role of neighbouring groups in enzymatic and carcinogenic action. Nature. 1966 Jan 8;209(5019):148–151. doi: 10.1038/209148a0. [DOI] [PubMed] [Google Scholar]
- Sparkes B. G., Walker I. G. Acid-soluble sulfhydryl compounds in L-cells during various conditions of growth. Can J Biochem. 1966 Aug;44(8):1159–1169. doi: 10.1139/o66-133. [DOI] [PubMed] [Google Scholar]
- Swann P. F., Magee P. N. Nitrosamine-induced carcinogenesis. The alklylation of nucleic acids of the rat by N-methyl-N-nitrosourea, dimethylnitrosamine, dimethyl sulphate and methyl methanesulphonate. Biochem J. 1968 Nov;110(1):39–47. doi: 10.1042/bj1100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher C. J., Walker I. G. Sensitivity of confluent and cycling embryonic hamster cells to sulfur mustard, 1,3-bis(2-chloroethyl)-1-nitrosourea, and actinomycin D. J Natl Cancer Inst. 1969 Mar;42(3):363–368. [PubMed] [Google Scholar]


