Abstract
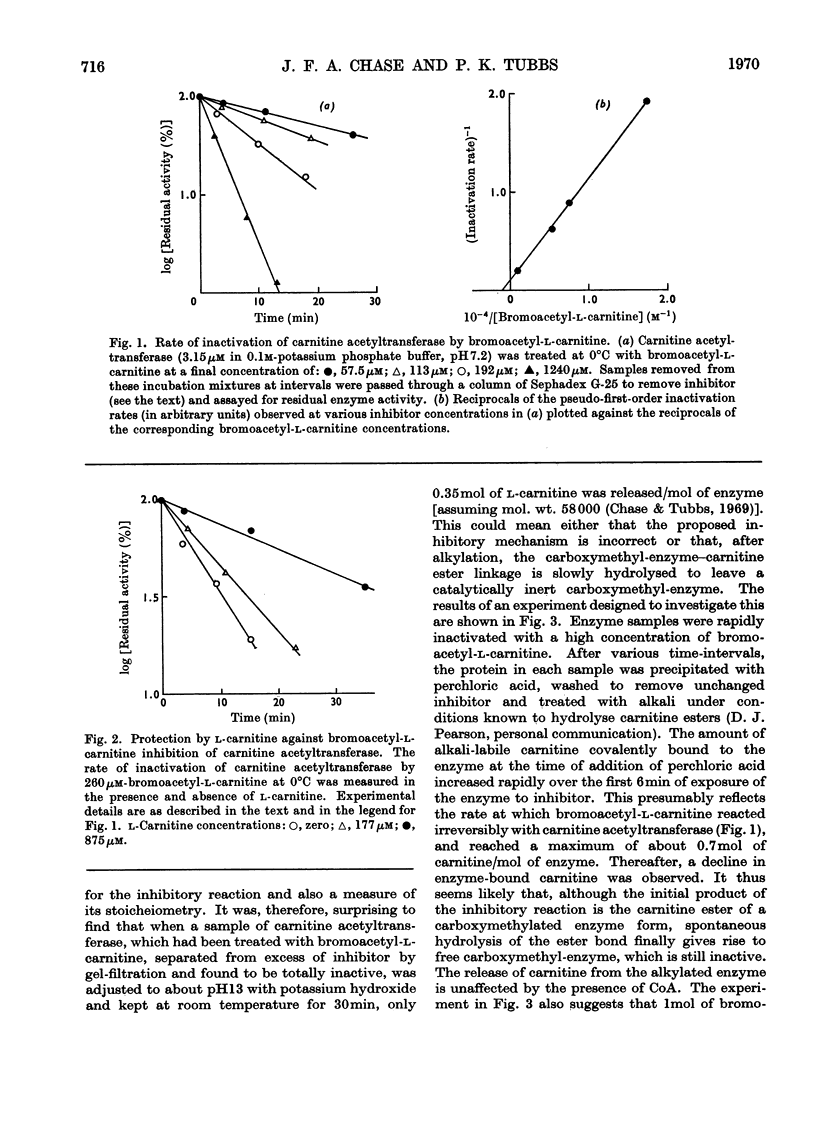
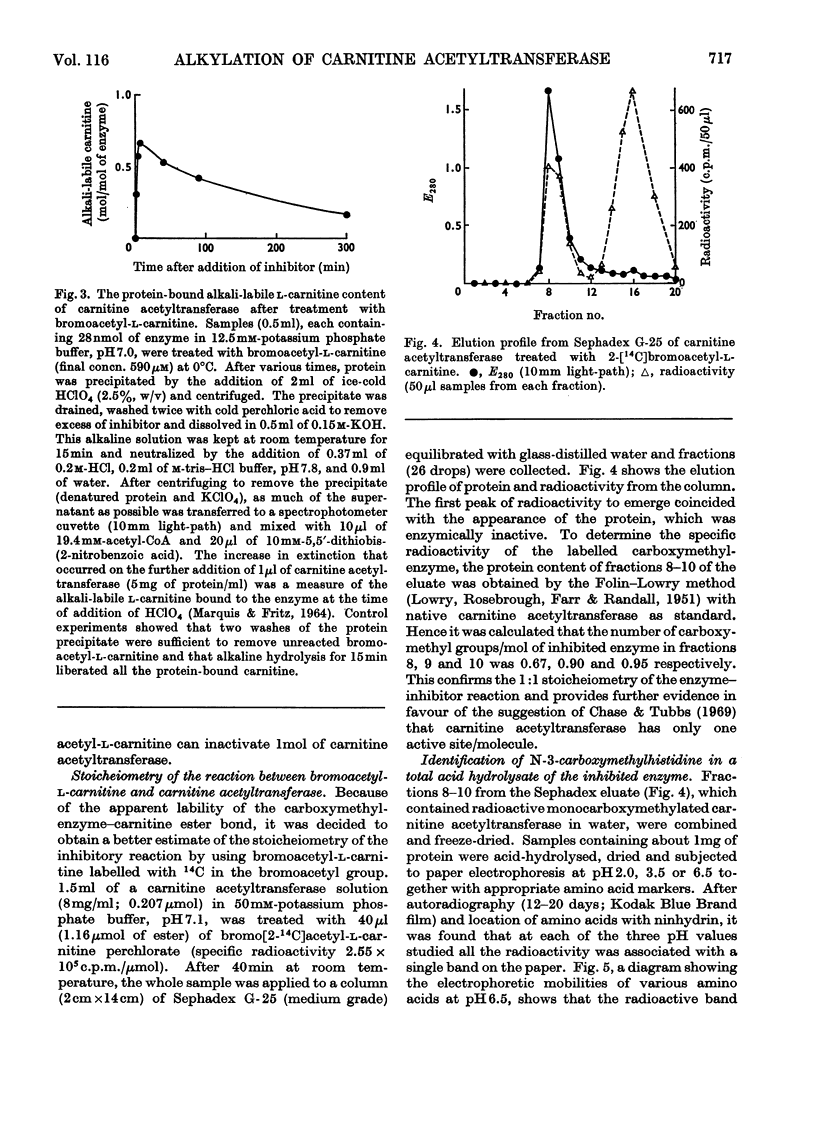
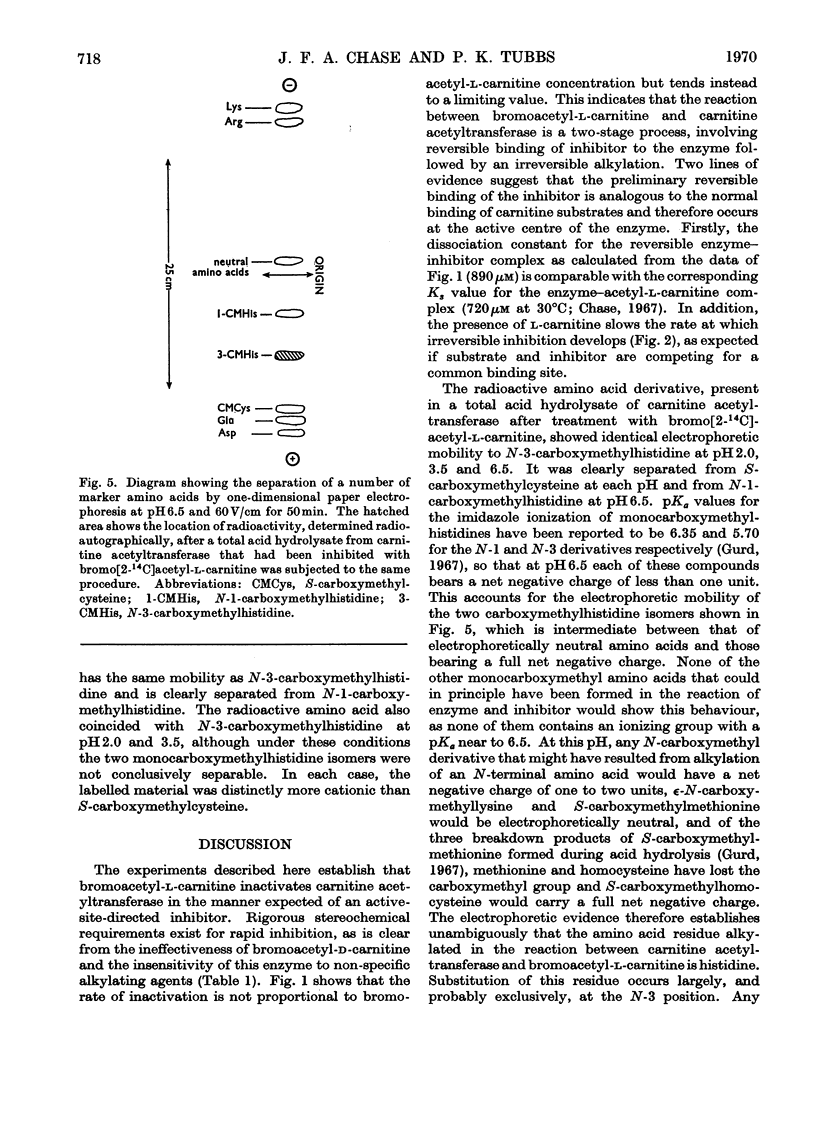
Incubation of carnitine acetyltransferase with low concentrations of bromoacetyl-l-carnitine causes a rapid and irreversible loss of enzyme activity; one mol of inhibitor can inactivate one mol of enzyme. Bromoacetyl-d-carnitine, iodoacetate or iodoacetamide are ineffective. l-Carnitine protects the transferase from bromoacetyl-l-carnitine. Investigation shows that the enzyme first reversibly binds bromoacetyl-l-carnitine with an affinity similar to that shown for the normal substrate acetyl-l-carnitine; this binding is followed by an alkylation reaction, forming the carnitine ester of a monocarboxymethyl-protein, which is catalytically inactive. The carnitine is released at an appreciable rate by spontaneous hydrolysis, and the resulting carboxymethyl-enzyme is also inactive. Total acid hydrolysis of enzyme after treatment with 2-[14C]bromoacetyl-l-carnitine yields N-3-carboxy[14C]methylhistidine as the only labelled amino acid. These findings, taken in conjunction with previous work, suggest that the single active centre of carnitine acetyltransferase contains a histidine residue.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHASE J. F., PEARSON D. J., TUBBS P. K. THE PREPARATION OF CRYSTALLIN CARNITINE ACETYLTRANSFERASE. Biochim Biophys Acta. 1965 Jan;96:162–165. doi: 10.1016/0005-2787(65)90622-2. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., STEIN W. H., MOORE S. Alkylation and identification of the histidine residues at the active site of ribonuclease. J Biol Chem. 1963 Jul;238:2413–2419. [PubMed] [Google Scholar]
- CRESTFIELD A. M., STEIN W. H., MOORE S. Properties and conformation of the histidine residues at the active site of ribonuclease. J Biol Chem. 1963 Jul;238:2421–2428. [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Conditions for the self-catalysed inactivation of carnitine acetyltransferase. A novel form of enzyme inhibition. Biochem J. 1969 Jan;111(2):225–235. doi: 10.1042/bj1110225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Some kinetic studies on the mechanism of action of carnitine acetyltransferase. Biochem J. 1966 Apr;99(1):32–40. doi: 10.1042/bj0990032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F. pH-dependence of carnitine acetyltransferase activity. Biochem J. 1967 Aug;104(2):503–509. doi: 10.1042/bj1040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN S., FRAENKEL G. Reversible enzymatic acetylation of carnitine. Arch Biochem Biophys. 1955 Dec;59(2):491–501. doi: 10.1016/0003-9861(55)90515-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARQUIS N. R., FRITZ I. B. ENZYMOLOGICAL DETERMINATION OF FREE CARNITINE CONCENTRATIONS IN RAT TISSUES. J Lipid Res. 1964 Apr;5:184–187. [PubMed] [Google Scholar]
- Perham R. N. A diagonal paper-electrophoretic technique for studying amino acid sequences around the cysteine and cystine residues of proteins. Biochem J. 1967 Dec;105(3):1203–1207. doi: 10.1042/bj1051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petra P. H., Cohen W., Shaw E. N. Isolation and characterization of the alkylated histidine from TLCK inhibited trypsin. Biochem Biophys Res Commun. 1965 Dec 21;21(6):612–618. doi: 10.1016/0006-291x(65)90530-9. [DOI] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- Shaw E., Ruscica J. The essentiality of histidine in the catalytic action of subtilisin. Covalent modification by a specific reagent. J Biol Chem. 1968 Dec 10;243(23):6312–6313. [PubMed] [Google Scholar]


