Abstract
Background/Objectives: The use of titanium meshes in bone regeneration is a clinical procedure that regenerates bone defects by ensuring graft stability and biocompatibility. The aim of the present investigation was to evaluate the clinical effectiveness of titanium mesh procedures in terms of vertical bone gain and the exposure rate. Methods: The product screening and eligibility analysis were performed using the Pubmed/MEDLINE, EMBASE, and Google Scholar electronic databases by two authors. The selected articles were classified based on the study design, regenerative technique, tested groups and materials, sample size, clinical findings, and follow-up. A risk of bias calculation was conducted on the selected randomized controlled trials (RCTs) and non-randomized trials and a series of pairwise meta-analysis calculations were performed for the vertical bone gain (VBG) and exposure rate. A significantly lower exposure rate was observed using coronally advanced lingual flaps (p < 0.05). No difference was observed between the titanium mesh and GBR techniques in terms of VBG (p > 0.05). Results: The initial search output 288 articles, and 164 papers were excluded after the eligibility analysis. The descriptive synthesis considered a total of 97 papers and 6 articles were considered for the pairwise comparison. Conclusions: Within the limits of the present investigation, the titanium mesh procedure reported high VBG values after the healing period. The mesh exposure rate was drastically lower with passive management of the surgical flap.
Keywords: bone regeneration, titanium mesh, jaws defects
1. Introduction
The treatment of severe bone ridge atrophies represents a complex clinical challenge in oral surgery due to the dysmorphic alteration of the oral tissues and the loss of support for implant rehabilitation [1,2,3]. Alveolar bone defects are commonly due to the loss of teeth, and from dentofacial traumas, neoplasms, and cyst expansion and removal defects, while the resorption rate could be severely increased other factors including infections and passive loading by an incongruous prosthesis [4,5]. On the other hand, different resorption patterns have been described between the horizontal and vertical components of the bone ridges of both the mandibular and maxillary ridges [2,4,5,6,7]. For this purpose, several different bone augmentation procedures for increasing the bone volume have been purposed including inlay/onlay bone grafts, bone distraction, guided bone regeneration, and titanium meshes [8,9,10,11]. Guided bone augmentation procedures are accomplished using the creation of a regenerative space based on scaffold positioning to stabilize the blood clot [12]. The addition of a covering made of collagen membranes has been used to compartmentalize the oral tissue components to restore the oral tissues’ anatomical morphology [6,13]. In the literature, this is described as the application of a non-resorbable membrane (i.e., polytetrafluoroethylene (PTFE)) or resorbable membrane (i.e., collagen) [14,15,16]. Historically, non-resorbable and titanium-reinforced membranes were used for guided bone regeneration procedures in the late 1980s due to their high mechanical stability and ability to maintain spaces [17]. Limitations of this technique include the necessity for a second surgery to remove the mesh and the tendency for exposure during the healing period [17]. Titanium meshes are a space-making device that have been used for the treatment of complex vertical defects due to the addition of a bone graft [11]. The theoretical advantage of titanium meshes is the presence of pores, which are able to create a favorable environment for the vascular sustenance and integration of the core graft [11,18]. Another interesting characteristic is the documented high biocompatibility of titanium, which prevents foreign body reactions and reduces the failure rate of the procedure [11,19]. Mechanically, the mesh is characterized by a high ductility due to the adaptation of the device to the bone defect area [11,19]. In addition, the device rigidity is able to guarantee a regenerative space during the healing phase and the graft integration process [11,19]. In the literature, the customization of titanium meshes through CAD/CAM has been used to increase the stability of regenerative devices and the on-chair procedure duration [20,21,22]. However, titanium meshes are technically sensitive and they are not free from complications. In fact, the main source of titanium mesh failure is from exposure of the mesh during the healing period, combined with contamination of the bone graft, which often irreversibly compromises the regenerative procedure [23]. The aim of the present systematic review was to investigate the clinical effectiveness of titanium meshes in bone regeneration procedures.
2. Materials and Methods
2.1. Screening of Scientific Articles
The literature search was performed following the criteria of the PICO guidelines (population, intervention, comparison, and outcome), as shown in Table 1. The data collected from the systematic search were processed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The present review was registered in the PROSPERO database (CRD42024585970). The Boolean search was carried out according to the strategy described in Table 2 and performed on the PubMed, EMBASE, and Google Scholar electronic databases (10 June 2024).
Table 1.
Summary of the PICO (population, intervention, comparison, and outcome) model.
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Subjects affected by severe bone ridge atrophy and are candidates for a graft | -Titanium mesh regeneration procedure | -Bone regeneration with a resorbable membrane | -Vertical bone gain -Mesh exposure |
Table 2.
Screening strategy using Boolean search.
| Search Strategies | |
|---|---|
| Keywords | (“titanium mesh” OR “titanium frames” OR “titanium membranes”) AND (“bone augmentation” OR “bone regeneration” OR “guided bone regeneration” OR ”bone-defect”) |
| Databases | PubMed/Medline, EMBASE, and Google Scholar |
2.2. Inclusion and Exclusion Criteria
In the initial screening phase, the identified studies were assessed based on the following inclusion criteria: human clinical trials, prospective or retrospective studies, case series or case reports, with no restriction on follow-up after surgical procedures or regarding the type of graft material mix used, and finally English-language papers.
The exclusion criteria were systematic literature reviews, editorial letters, in vitro studies, and animal studies. The evaluation of the manuscripts using the above criteria was performed for the purpose of including them in the eligibility analysis.
2.3. Screening Process
Two expert reviewers (FL and IA) independently and blindly performed the selection and screening of articles in order to identify scientific articles for the analysis processes. However, the articles that were excluded from the research work according to the criteria are reported in the paper as well as the justification for their exclusion.
2.4. Data Analysis
A database was specifically created using Excel software (Microsoft, Redmond, WA, USA) to enter the data collected from the included scientific studies. The collected data were classified according to the following characteristics: study design, sample size, regenerative technique, complications, biomaterial/resorbable membrane type, surgical flap technique, and follow-up.
2.5. Outcome Measures
The outcome measures considered for the data analysis were the occurrence of flap exposure during the bone regeneration healing period (<6 months), and the vertical bone height and horizontal bone gains calculated at the follow-up using computed tomography assessments.
2.6. Risk of Bias Assessment (RoB)
An RoB analysis was performed according to the OHAT Guidelines and Risk of Bias Rating Tool for Human and Animal Studies using Rev Man 5.5 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014). Only the trials included for the meta-analysis process were submitted to the risk of bias assessment [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. The following guideline criteria were applied: randomization sequence, allocation concealment, blinding participants, blinding outcomes, incomplete outcome data, selective reporting, and other biases. The RoB parameters were classified as adequate, unclear, or inadequate. The minimum RoB ratio was a total of 5/7 low risk (lr) indicators with/without unclear risk (ur) parameters. Otherwise, the articles were categorized as high risk (hr).
2.7. Meta-Analysis
A forest plot of the relative effects was generated to assess the consistency and the significance of the rankings. I2 < 40% was considered low heterogeneity. To guarantee valid pairwise comparisons, we selected studies with similar methodologies for further statistical calculations. Pairwise comparisons were performed for the titanium mesh group vs. membrane regeneration group and the coronally advanced lingual flap group vs. control group considering the site exposure and vertical bone gain (VBG) parameters. The exposure rate was expressed as an Odds Ratio (OR) and the VBG was expressed as the mean difference (MD).
3. Results
3.1. Screening Procedure
The search conducted using electronic databases (PubMed/Medline, EMBASE, and Google Scholar) found 288 articles. The search did not detect duplicates so the scientific articles were submitted for eligibility evaluation. A total of 164 articles were excluded from the synthesis process for reasons such as being off-topic (114 articles), being in a different language (15 publications), using an animal model (41 scientific articles), and being a literature review (21 papers). As a result of the careful selection, a total of 97 scientific articles were included in the descriptive analysis and 6 articles were considered for the pairwise meta-analysis. This systematic literature review included retrospective case–control studies, prospective studies, cohort studies, case series and case reports, randomized controlled trials, non-RCTs, preliminary studies, and comparative studies (Figure 1; Table 2).
Figure 1.
Screening flowchart for the investigated studies following the PRISMA guidelines. ** the step was performed by human with no automation tools.
3.2. Characteristics of the Included Studies
The descriptive synthesis reported that the most frequent grafts used for bone regeneration were autogenous bone and autogenous bone mixed with a heterologous bone graft. Some studies differed in the autogenous/heterologous mix ratio, which ranged from a ratio of 50:50 to 70:30 of autogenous bone and BBM (bovine bone mineral). The combination of platelet-rich plasma (PRP), collagen sponges (rhBMP-2 ÷ ACS), resorbable collagen membranes, and alloplastic materials mixed with a nano-bone graft was also reported (Table 3 and Table 4). The most frequent complication reported was mesh exposure that was correlated to a partial failure of the graft or, in some cases, a higher incidence of compromised bone grafts. Other reported complications were infection, total/partial bone resorption, temporary neurological disturbances, and implant failure. The follow-up results were heterogeneous since the follow-up time in the included studies ranged from 5 months to 20.5 years (Table 3 and Table 4).
Table 3.
Studies included after the literature screening [RCT: randomized controlled trial; non-RCT: non-randomized controlled trial]. The synthesis was performed considering the regenerative methods, study model design, sample size, and test and control groups.
| First Author | Journal | Year | Methods | Study Design | Sample Size | Test Group | Control Group |
|---|---|---|---|---|---|---|---|
| Boyne PJ [24] | Head Neck Surg | 1983 | Ti mesh | Case series | 6 patients | 6 cases after neoplastic resection | - |
| L Malchiodi [25] | Int J Oral & Maxillofacial Imp | 1998 | Ti mesh | Case report | 25 patients | - | - |
| von Arx T [26] | Int J Periodontics Restorative Dent | 1998 | Ti mesh | Case report | 6 patients | 10 implant sites | - |
| von Arx T [27] | Clin Oral Implants Res | 1999 | Ti mesh/microscrews | Non-RCT | 15 patients | 20 implants placed in GBR sites | - |
| Sumi Y [28] | Oral Surg Oral Med Oral Pathol Oral Radiol Endod | 2000 | Ti mesh/bone screws | Case report | 3 patients | Implant placement into 3 sites | - |
| Klug CN [29] | J Oral Maxillofac Surg | 2001 | Ti mesh (microscrews), distractor | Case series | 10 patients | Intraoral microplate distractors for severe atrophy of edentulous molar region placed in 13 sites | - |
| Maiorana C [30] | Int J Oral Maxillofac Implants | 2001 | Ti mesh | Non-RCT | - | - | - |
| Artzi Z [31] | Int J Oral Maxillofac Implants | 2003 | Ti mesh (screws) | Case report | 10 patients | 10 severely resorbed sites in root(screw-type implants) | - |
| Degidi M [32] | J Oral Implantol | 2003 | Ti mesh | Non-RCT | 18 patients | ||
| Proussaefs P [33] | Int J Periodontics Restorative Dent | 2003 | Ti mesh | Non-RCT | 7 patients | 7 surgical sites treated with titanium mesh and graft | - |
| Roccuzzo M [34] | Clin Oral Implants Res | 2004 | Ti mesh | Case report | 18 patients | Ti mesh with biomaterial fixed using titanium screws | - |
| Proussaefs P [35] | J Oral Implantol | 2006 | Ti mesh | Non-RCT | 17 patients | Ti mesh with 50:50 autogenous graft/Bio-Oss | - |
| Kfir E [36] | J Oral Implantol | 2007 | Ti mesh | Case report | 15 patients | Ti mesh with biomaterial | - |
| Longoni S [37] | The International journal of oral & maxillofacial implants | 2007 | Ti mesh | Case report | 1 patient | Ti mesh with biomaterial | - |
| Roccuzzo M [38] | Clin Oral Implants Res | 2007 | Ti mesh | RCT | 23 patients | Bone graft + Ti mesh at 12 sites | 12 sites (bone graft alone) |
| Aikawa T [39] | Oral Surg Oral MedOral Pathol Oral Radiol Endod | 2008 | Ti mesh plate | Case report | 1 patient | Defect due to keratocystic odontogenic extirpation (15 yrs before) | - |
| Pieri F [40] | J Periodontol | 2008 | Ti mesh | Clinical trial | 16 patients | 19 reconstructive procedures with delayed implant (44) | - |
| Corinaldesi G [41] | Int J Oral Maxillofac Implants | 2009 | Ti mesh | Retrospective study | 24 patients | 13 Patients: 20 implants placed using reconstructive procedure | 11P: 36 implants, second surgery 8 to 9 months later |
| Torres J [42] | J Clin Periodontol | 2010 | Ti mesh | RCT | 30 patients | 15 P: Ti meshes covered with PRP | 15P: Ti meshes with no PRP |
| Ciocca L [43] | Med Biol Eng Comput | 2011 | Customized Ti mesh Direct metal laser sintering (DMLS) |
Case report | 1 patient | A 53-yo M subject treated with 3D customized titanium mesh | - |
| Misch CM [44] | Int J Periodontics Restorative Dent. | 2011 | Ti mesh | Case report | 5 patients | Ti mesh + rhBMP-2 ÷ ACS | - |
| Cicciù M [45] | Open Dent J | 2012 | Ti mesh plate, monocortical screws |
Case report | 1 patient | Defects associated with dentinogenic ghost cell tumor; Ti plate and mesh + absorbable collagen sponge + rhBMP-2 | - |
| Her S [46] | J Oral Maxillofac Surg | 2012 | Ti mesh, titanium screws |
Retrospective study | 26 patients | 27 sites: bone grafting + fixation of titanium mesh | - |
| Miyamoto I [47] | Clin Implant Dent Relat Res | 2012 | Ti mesh | - | 41 patients | 50 surgical sites | - |
| Ciocca L [48] | Comput Methods Biomech Biomed Engin | 2013 | Ti mesh, titanium screws |
Case report | 1 patient | Bone defect in a 46-yo male subject | - |
| Funato A [49] | Int J Periodontics Restorative Dent | 2013 | Ti mesh | Retrospective study | 19 patients | - | - |
| Atef M [50] | Clin Implant Dent Relat Res. | 2014 | Ti mesh | Case series | 4 patients | 8 maxillary sinus sites treated with titanium mesh elevation | - |
| Butura CC [51] | Int J Oral Maxillofac Implants | 2014 | Ti mesh Rigid screw fixation |
Case report | 7 patients | 23 compromised alveolar sites underwent extraction and debridement | - |
| Jung GU [52] | J Korean Assoc Oral Maxillofac Surg | 2014 | Ti mesh | Preliminary study | 10 patients | Sites treated with biomaterial covered by Ti mesh | - |
| Katanec D [53] | Coll Antropol | 2014 | Ti mesh-retaining screws | Case report | 61 patients | - | - |
| Levine RA [54] | Compend Contin Educ Dent | 2014 | Ti mesh | Case report | 1 patient | Single implant in premolar region | - |
| Lizio G [55] | Int J Oral Maxillofac Implants | 2014 | Ti mesh | Retrospective study | 12 patients | 15 alveolar defects treated with Ti mesh and particulate grafts | - |
| Poli PP [56] | Open Dent J | 2014 | Ti mesh | Retrospective clinical study | 13 patients | Ti mesh filled with intraoral biomaterial | - |
| Vrielinck L [57] | J Craniofac Surg | 2014 | Custom-made titanium membrane | Case Report | 1 patient | Odontogenic keratocyst with remaining inferior alveolar nerve removed and curettage of the lesion; Ti plate fixed with screws | - |
| De Angelis N [58] | J Periodontics Restorative Dent | 2015 | Pre-adapted Ti mesh bone screws |
Case series | 2 patients | Surgical site: Ti mesh + rhPDGF | - |
| Di Stefano DA [59] | J Contemp Dent Pract | 2015 | Pre-shaped Ti mesh | Case report | 1 patient | Titanium mesh graft treatment in a 54-yo patient | - |
| Kim Y [60] | Dent Traumatol | 2015 | Ti mesh | Case report | 3 patients | Ti mesh + biomaterial + membrane | - |
| Lee JT [61] | J Korean Assoc Oral Maxillofac Surg | 2015 | Ti mesh | Case report | 1 patient | 1. Failed intra-mobile cylinder implant system 2. Failed Ti mesh 3. Distraction osteogenesis |
- |
| Sumida T [62] | J Craniomaxillofac Surg | 2015 | Ti mesh-retaining screws | Non-RCT | 26 patients | 13 patients: custom-made devices | Commercial Ti mesh that was bent during operation |
| Misch CM [63] | Int J Oral Maxillofac Implants | 2015 | Pre-shaped Ti mesh | Retrospective study | 1 patient | Titanium mesh graft treatment in 54-yo patient | - |
| Knöfler W [64] | Int J Implant Dent | 2016 | Ti mesh membranes as graft materials | Retrospective study | 3095 patients | Titanium mesh in augmented sites | No augmented sites |
| Zita Gomes R [65] | Biomed Res Int | 2016 | Ti mesh for horizontal ridge defect | RCT | 25 patients | 40 implants with simultaneous GBR and Ti meshes | - |
| Ahmed M [66] | Int J Oral Maxillofac Surg | 2017 | Micro- (0.1 mm) and resorbable (0.3 mm poly-dl-lactide) Ti meshes | Case series, split-mouth study |
8 patients | Bilateral sinus pneumatization sites; lateral window technique (in sinuses); elevated and maintained with resorbable membrane | Elevated and maintained with Ti meshes |
| Cucchi A [67] | Clin Implant Dent Relat Res | 2017 | Ti mesh | RCT | 40 patients | Titanium mesh graft | PTFE-reinforced membrane |
| Jegham H [68] | J Stomatol Oral Maxillofac Surg | 2017 | Customized Ti mesh to shape fixing screws |
Case report | 1 patient | 1 surgical site for implant in maxillary central incisor | - |
| Scarano A [69] | Oral Implantol (Rome) | 2017 | Ti mesh | Case report | 3 patients | 3 implant defects | - |
| Alagl AS [70] | J Int Med Res | 2018 | Ti mesh | Case report | 1 patient | Regeneration site implant at central incisor position | - |
| Ciocca L [71] | J Oral Implantol | 2018 | Ti mesh (CAD-CAM-customized) -retaining screws | Non-RCT | 9 patients | Implant surgery at atrophic sites | - |
| Inoue K [72] | Implant dentistry | 2018 | Selective laser melting titanium mesh sheet | Case series | 2 patients | Laser melt titanium mesh/immediate implant | - |
| Lorenz J [73] | J Oral Implantol | 2018 | Ti mesh (3D planned) | Case report | 1 patient | 1 surgical site in a squamous cell carcinoma patient | - |
| Zhou M [74] | J Oral Implantol | 2018 | Ti mesh | Case report | 1 patient | Bone deficiency in the No. 11 and No. 24–25 regions | - |
| Cucchi A [75] | J Oral Implantol | 2019 | Custom-made CAD/CAM titanium meshes | Case report | 1 patient | Custom-made titanium mesh/immediate implant | - |
| Di Stefano DA [59] | Dent J (Basel) | 2019 | Pre-shaped Ti mesh | Case report | 1 patient | GBR/implant position | - |
| Hartmann A [76] | Implant Dent | 2019 | Titanium mesh | Non-RCT | 65 patients | Titanium mesh | - |
| Mounir M [77] | Clin Implant Dent Relat Res | 2019 | Ti mesh and customized poly-ether-ether-ketone (PEEK) mesh |
RCT | - | ||
| Tallarico M [78] | Materials (Basel) | 2019 | Ultra-fine titanium mesh | Case series | 7 patients | Ultra-fine titanium mesh/immediate implant | - |
| Zhang T [79] | Clin Implant Dent Relat Res | 2019 | L-shaped titanium mesh | Retrospective study | 12 patients | L-shaped titanium mesh | - |
| Atef M [6] | Clin Implant Dent Relat Res | 2020 | Titanium mesh | RCT | 20 patients | Titanium mesh | Collagen membrane |
| Hartmann A [80] | BMC Oral Health | 2020 | Customized titanium mesh | Retrospective study | 98 patients | Titanium mesh | |
| Maiorana C [81] | Materials (Basel, Switzerland) | 2020 | Titanium meshes | Pilot study | 8 patients | Peri-implant defects treated with titanium mesh | - |
| Malik R [82] | J Maxillofac Oral Surg | 2020 | Titanium mesh | Non-RCT | 16 patients | Titanium mesh | - |
| Tallarico M [83] | Materials (Basel, Switzerland) | 2020 | 3D titanium meshes | Case report | 1 patient | 3D titanium meshes | - |
| Li L [84] | Clin Implant Dent Relat Res | 2021 | 3D titanium meshes | Retrospective study | 16 patients | 3D titanium meshes | - |
| Chiapasco M [85] | Clin Oral Implants Res | 2021 | CAD/CAM titanium mesh | Retrospective study | 41 patients | CAD/CAM titanium mesh | - |
| Cucchi A [86] | Int J Oral Implantology | 2021 | Titanium mesh | RCT | 40 patients | Titanium mesh | PTFE-reinforced membrane |
| Cucchi A [87] | Clin Oral Implants Res | 2021 | Custom-made CAD/CAM titanium meshes | RCT | 30 patients | Titanium mesh with resorbable membranes | Titanium mesh without resorbable membranes |
| De Santis D [88] | Medicina (Kaunas) | 2021 | Digital Customized Titanium Mesh | Case series | 5 patients | Custom-made CAD/CAM titanium meshes | - |
| Dellavia C [89] | Clin Implant Dent Relat Res | 2021 | Custom-made CAD/CAM titanium meshes | Cohort study | 20 patients | Custom-made CAD/CAM titanium meshes | - |
| Kadkhodazadeh M [90] | Oral Maxillofac Surg | 2021 | Titanium meshes | Pilot study | 7 patients | Titanium mesh with resorbable membranes | - |
| Lee SR [13] | Materials (Basel, Switzerland) | 2021 | 3D titanium meshes | RCT | 28 patients | Titanium mesh with cross-linked collagen membrane | Titanium mesh with non-cross-linked collagen membrane |
| Li S [91] | Int J Oral Sci | 2021 | Digital titanium mesh | Non-RCT | 40 patients | Digital titanium mesh | Resorbable membranes |
| Maiorana C [92] | J Contemp Dent Pract | 2021 | Titanium meshes | Non-RCT; split-mouth study | 5 patients | Titanium mesh | Dense polytetrafluoroethylene membrane |
| Wang X [93] | Biomed Res Int | 2021 | Titanium mesh membranes CGF membranes |
Non-RCT | 18 patients | Titanium mesh membranes and CGF membranes | - |
| Yoon JH [94] | Maxillofac Plast Reconstr Surg | 2021 | Titanium mesh | Case report | 1 patient | Titanium mesh and pedicled buccal fat pad | - |
| Bertran Faus A [95] | Materials (Basel) | 2022 | Custom-made CAD/CAM titanium meshes | Case report | 1 patient | Custom-made titanium mesh | - |
| Boogaard MJ [20] | Compend Contin Educ Dent | 2022 | Custom-made CAD/CAM titanium meshes | Case series | 2 patients | Custom-made titanium mesh | - |
| Del Barrio RAL [96] | J Oral Implantol | 2022 | Titanium mesh frame (TF) | Case report | 1 patient | Titanium mesh combined with recombinant human bone morphogenetic protein-2, deproteinized bovine bone mineral | |
| Gelețu GL [97] | Medicina (Kaunas) | 2022 | Custom-made CAD/CAM titanium meshes | Case report | 1 patient | Custom-made titanium mesh | - |
| Hartmann A [98] | Clin Oral Implants Res | 2022 | Titanium mesh frame (TF) | Non-RCT | 21 patients | Bone regeneration (GBR)/titanium mesh (TM) | - |
| Levine RA [99] | Int J Periodontics Restorative Dent | 2022 | Titanium mesh frame (TF) | Retrospective study | 48 patients | Ti mesh guided bone regeneration | - |
| Lim J [100] | Materials (Basel) | 2022 | Titanium mesh frame (TF) | RCT | 18 patients | Inorganic bovine bone materials (Bio-Oss) | A-Oss xenograft (Osstem, Seoul, Korea), |
| Majewski P [101] | Int J Periodontics Restorative Dent | 2022 | Titanium mesh frame (TF) | Case series | 6 patients | Bone regeneration (GBR)/titanium mesh (TM) | - |
| Müller J [102] | Case Rep Dent | 2022 | Titanium mesh frame (TF) | Case report | 1 patient | CAD CAM Ti mesh guided bone regeneration with previous bisphosponate treatment | - |
| Poomprakobsri K [103] | J Oral Implantol | 2022 | Titanium mesh frame (TF)/fixation screws | Retrospective study | - | Group 1: resorbable barrier Group 2: non-resorbable barrier Group 3: titanium-mesh barrier |
- |
| Yang W [104] | BMC Oral Health | 2022 | Titanium mesh frame (TF) | non-RCT | 20 patients | Defect volume < 150 mm2 | Defect volume > 150 mm2 |
| Abaza AWAAB [3] | Int J Oral Maxillofac Implants | 2023 | Titanium mesh frame (TF) | RCT | 38 patients | Bone regeneration (GBR)/3D-printed individualized titanium mesh (3D-PITM) | Collagen group |
| Attia R [105] | Int J Periodontics Restorative Dent | 2023 | Titanium mesh frame (TF) | RCT | 14 patients | Bone regeneration/coronally advanced lingual flap (CALF) | Bone regeneration/no coronally advanced lingual flap (CALF) |
| Bahaa S [106] | Int J Oral Maxillofac Surg | 2023 | Titanium mesh frame (TF) | RCT | 40 patients | Flap groups: -Incision (PRI) -Double flap incision (DFI) -Modified periosteal releasing incision (MPRI) -Coronally advanced lingual flap (CALF) |
- |
| Chen D [21] | Clin Implant Dent Relat Res | 2023 | CAD/CAM titanium mesh frame (TF) | Retrospective study | 30 patients | Screw-position-guided template | No screws for position-guided template |
| Kurtiş B [22] | J Oral Implantol | 2023 | CAD/CAM titanium mesh frame (TF) | Case report | 1 patient | Vertical bone augmentation/titanium mesh | - |
| Nan X [107] | Clin Oral Implants Res | 2023 | CAD/CAM titanium mesh frame (TF) | Retrospective study | 59 patients | Bone regeneration (GBR)/3D-printed individualized titanium mesh (3D-PITM) | - |
| Onică N | Healthcare (Basel) | 2023 | CAD/CAM titanium mesh frame (TF) | Case report | 1 patient | Bone regeneration (GBR)/3D-printed individualized titanium mesh (3D-PITM) | - |
| Onodera K [108] | J Clin Med | 2023 | Titanium mesh frame (TF) for severe mandibular bone defects | Retrospective study | 18 patients | Custom-made titanium mesh | - |
| Songhang Li [109] | Clin Implant Dent Relat Res | 2023 | Titanium mesh frame (TF) | Retrospective study | 36 patients | Titanium mesh stabilized with resorbable sutures | Titanium mesh stabilized with titanium screws |
| Wen SC [110] | Int J Periodontics Restorative Dent | 2023 | Titanium mesh frame (TF) | Case series | 3 patients | Pre-trimmed TFs/graft and membrane | Pre-trimmed TFs/graft, no collagen membrane |
| Zhang G [111] | J Esthet Restor Dent | 2023 | Titanium mesh frame (TF) | Case series | 3 patients | Tooth-supported TFs | - |
Table 4.
Studies included after the literature screening. The synthesis was performed considering the technique, complications, bone graft study outcome, findings, and follow-up.
| First Author | Journal | Year | Technique | Complications | Biomaterial/Membrane Type | Outcome | Follow-Up |
|---|---|---|---|---|---|---|---|
| Boyne PJ [24] | Head Neck Surg | 1983 | Titanium mesh | Patient #1: additional graft due to insufficient gain | - | Regenerated residual mandible segments from hemi-mandibulectomy | 8–12 years |
| L Malchiodi [25] | Int J Oral & Maxillofacial Imp | 1998 | Titanium mesh | Patient #1: dehiscences around 3 implants | Autogenous bone graft | Higher width for alveolar ridge (mean: 5.65 mm; range: 5.20–6.10 mm) | 8 months |
| von Arx T [26] | Int J Periodontics Restorative Dent | 1998 | Titanium mesh | None | Autogenous bone graft | All sites successfully treated | - |
| von Arx T [27] | Clin Oral Implants Res | 1999 | Titanium mesh | Implant complications (8): exposure (~6.5 mm), dehiscences (80%), fenestrations (20%), mesh exposure (rate: 5%) |
Autogenous bone graft | Mean vertical bone height: 5.8 mm Mean bone defect filling: 93.5% |
6.6 months |
| Sumi Y [28] | Oral Surg Oral Med Oral Pathol Oral Radiol Endod | 2000 | Titanium mesh | None | Autogenous bone graft | Regenerated alveolar crest width: 6.5 mm to 8 mm Mean gain in crest width: 3.5 mm |
6 to 9 months |
| Klug CN [29] | J Oral Maxillofac Surg | 2001 | Titanium mesh L-shaped osteotomy |
Patient #1: distractor fracture Patient #2: dehiscence |
- | Mean bone height: 7.5 mm | 19 months |
| Maiorana C [30] | Int J Oral Maxillofac Implants | 2001 | Titanium mesh | None | Autogenous cancellous bone/Bio-Oss in 1:1 ratio | Augmentation procedure showed bone regeneration and the presence of vessels, indicating bone vitality | 5 to 6 months |
| Artzi Z [31] | Int J Oral Maxillofac Implants | 2003 | Titanium mesh | None | Xenograft | Defect height: Before = 6.4 +/− 1.17 mm After = 1.2 mm +/− 0.63 Bone height: 5.2 +/− 0.79 mm Average bone fill: 81.2% +/− 7.98 |
9 months |
| Degidi M [32] | J Oral Implantol | 2003 | Titanium mesh | None | Autogenous bone graft with bone-resorbable membrane | All cases had a good esthetic result after the restorative procedures | 7 years |
| Proussaefs P [33] | Int J Periodontics Restorative Dent | 2003 | Titanium mesh | Mesh exposure with no compromise of graft | Bio-Oss, autogenous bone graft | Ridge augmentation: 2.86 mm (vertical), 3.71 mm (horizontal) Bone grafted area: 36.4% Graft resorption: 15.08% |
6 months |
| Roccuzzo M [34] | Clin Oral Implants Res | 2004 | Titanium mesh | None | Autogenous bone graft, particulate xenograft |
Mean vertical bone augmentation: 4.8 mm (range: 4–7 mm) |
4.6 months |
| Proussaefs P [35] | J Oral Implantol | 2006 | Titanium mesh | Patient #2: early mesh exposure (2 weeks) Patient #4: latemesh exposure (>3 months) |
Autogenous bone graft and Bio-Oss in 50:50 ratio | 36.47% new bone formation 15.11% resorption |
12 months |
| Kfir E [36] | J Oral Implantol | 2007 | Titanium mesh | Patient #7: early membrane exposure (47%) | Autologous platelet-rich fibrin | Sufficient bone augmentation in 8P | 18 weeks |
| Longoni S [37] | Int J Oral & Maxillofacial Imp | 2007 | Titanium mesh | None | Regenaform demineralized freeze-dried bone allograft | - | 18 months |
| Roccuzzo M [38] | Clin Oral Implants Res | 2007 | Titanium mesh | None | Autogenous onlay bone graft | Vertical augmentation: 5 mm T.g., 3.4 mm T.c. bone resorption: 13.5% T.g., 34.5% T.c. |
4.6 months |
| Aikawa T [39] | Oral Surg Oral MedOral Pathol Oral Radiol Endod | 2008 | Ti mesh plate distraction device with titanium microscrews | None | None | 4 mm widening at the first molar region Wider alveolar ridge |
6 months |
| Pieri F [40] | J Periodontol | 2008 | Titanium mesh | 19 micro-meshes exposed after 2 months (5.3%) 3 implants removed, bone resorption > 2 mm |
70:30 mixture of autogenous bone graft and BBM (bovine bone mineral) |
Mean vertical augmentation 3.71–1.24 mm Mean horizontal augmentation 4.16–0.59 mm |
2 years |
| Corinaldesi G [41] | Int J Oral Maxillofac Implants | 2009 | Titanium mesh | 4 micro-meshes exposed and removed (complication rate: 14.8%) | Autogenous bone graft | Vertical bone augmentation: 5.4 +/− 1.81 mm T. group: 4.5 +/− 1.16 mm C. group: implant CSR: 96.4% |
3–8 years |
| Torres J [42] | J Clin Periodontol | 2010 | Titanium mesh | Control group: 28.5% mesh exposure Test group: none |
Inorganic bovine bone (ABB), platelet-rich plasma (PRP) | Bone augmentation greater in test group: 97.3% in control group, 100% in test group | 24 months |
| Ciocca L [43] | Med Biol Eng Comput | 2011 | Titanium mesh | - | Particulate autogenous bone graft, bovine demineralized bone | Mean vertical height difference in crestal bone: 2.57 mm Mean buccal–palatal increase: 3.41 mm |
8 months |
| Misch CM [44] | Int J Periodontics Restorative Dent. | 2011 | Titanium mesh | - | Recombinant human BMP 2 ÷ acellular collagen sponge (rhBMP-2 ÷ ACS) | All 10 implants integrated | 6 months |
| Cicciù M [45] | Open Dent J | 2012 | Titanium mesh | - | Absorbable collagen sponge, rhBMP-2 | Mandibular continuity was regained | 9 months |
| Her S [46] | J Oral Maxillofac Surg | 2012 | Titanium mesh | Ti mesh exposure rate: 26% | Puros-Bio-Oss autogenous graft | All 69 implants placed in function, 100% success rate | 6–24 months |
| Miyamoto I [47] | Clin Implant Dent Relat Res | 2012 | Titanium mesh | Mesh exposure, infection, total/partial bone resorption, temporary neurological disturbances 1 implant failure |
Autogenous particulate bone graft or iliac cancellous bone marrow grafts | Gain (mm): HV (H 3.7 ± 2.0; V 5.4 ± 3.4); H (3.9 ± 1.9) S > bone augmentation (H, 5.7 ± 1.4; V 12.4 ± 3.1) HV >> bone resorption (p < 0.05) |
9 years |
| Ciocca L [48] | Comput Methods Biomech Biomed Engin | 2013 | CAD/CAM titanium mesh | - | Particulate, autogenous, bovine demineralized bone | Bone necessary for implants regenerated, bone augmentation in right lingual region extended beyond planned augmentation, maximum deviation < 1.5 mm | 6 months |
| Funato A [49] | Int J Periodontics Restorative Dent | 2013 | Titanium mesh | Patient #1: flap dehiscence during healing period Patient #1: collagen membrane exposed during delayed period |
Resorbable collagen membrane (cover of Ti mesh) Autogenous bone graft, Inorganic bovine bone particles + * Recombinant human platelet-derived growth factor BB |
Mean vertical height of augmented bone: 8.6 ± 4.0 mm | 8.0 ± 1.4 months |
| Atef M [50] | Clin Implant Dent Relat Res. | 2014 | Ti micromesh in maxillary sinus | - | - | Residual ridge height: 3.6 ± 1.6 mm Ridge height at follow-up: 9.63 ± 1.47 mm Volume of native bone: 30.3% ± 9.1% Volume of new bone: 55.3% ± 11.4% |
6 months |
| Butura CC [51] | Int J Oral Maxillofac Implants | 2014 | Titanium mesh | - | rhBMP-2—inorganic bovine bone | Defects successfully regenerated with no additional surgery prior to implant placement or prosthetic restoration 14 implants placed and restored |
6 months |
| Jung GU [52] | J Korean Assoc Oral Maxillofac Surg | 2014 | Titanium mesh | None | Particulate intraoral autologous bone + freeze-dried bone allograft in 1:1 volume ratio |
New growth: 80% vital bone, 5% fibrous marrow tissue, 15% remaining allograft; all implants were functional | 16 months |
| Katanec D [53] | Coll Antropol | 2014 | Titanium mesh | None | BMPs administered via an absorbable collagen sponge carrier (ACS) used for bone induction | Bone vertical gain: 5.5 mm (on the left), 5 mm (on the right), with 6 mm width Implant stability quotient (ISQ): 69 -75 |
24 months |
| Levine RA [54] | Compend Contin Educ Dent | 2014 | Titanium mesh | - | - | - | 3 years |
| Lizio G [55] | Int J Oral Maxillofac Implants | 2014 | Titanium mesh | Mesh exposure occurred at 80% of augmented sites (0.73 cm2) at 2.17 months 16.3% LBV for every cm2 of mesh exposed |
70:30 autogenous bone graft/inorganic bovine bone | LBV (lacking bone volume)-PBV (planned bone volume) Mean LBV (0.45 cm3) was 30.2% of the mean PBV (1.49 cm3) |
8–9 months |
| Poli PP [56] | Open Dent J | 2014 | Titanium mesh | None | 1:1 ratio autogenous bone graft mix with deproteinized inorganic bovine bone | Mean peri-implant bone loss of 1.743 mm on mesial side, 1.913 mm on distal side, from the top of implant head to the first visible bone–implant contact |
88 months |
| Vrielinck L [57] | J Craniofac Surg | 2014 | Titanium mesh | No evidence of residual cyst | Xenograft | Restored mandibular shape and facial symmetry; promoted new bone formation to fill in the mandibular defects | 5 years |
| De Angelis N [58] | J Periodontics Restorative Dent | 2015 | Titanium mesh | None | rhPDGF-BB, inorganic bovine bone particles, equine collagen sponge | Enough bone was regenerated to plan the implant placement according to the initial prosthetic plan and the patient requests | 3 years |
| Di Stefano DA [59] | J Contemp Dent Pract | 2015 | Titanium mesh | None | 30:70 mixture of autogenous bone graft and equine, enzyme-deantigenic collagen-preserved bone substitute | At follow-up, implants were perfectly functional, and the bone width was stable over time | 24 months |
| Kim Y [60] | Dent Traumatol | 2015 | Titanium mesh | None | Xenograft + bone fragments from traumatic site Resorbable collagen membrane (on bone graft site) |
Sufficiently preserved alveolar bone for implant placement | 6 months |
| Lee JT [61] | J Korean Assoc Oral Maxillofac Surg | 2015 | Titanium mesh distraction osteogenesis |
Xenograft | - | ||
| Sumida T [62] | J Craniomaxillofac Surg | 2015 | Titanium mesh | Mucosal rupture (p = 0.27) in 1 patient with custom-made Ti mesh No severe infection (7.7%), 3 infections in control group (23.1%) |
None | Operation time (min) t. group: 75.4 ± 11.6 c. group: 111.9 ± 17.8 |
6 months |
| Misch CM [63] | Int J Oral Maxillofac Implants | 2015 | Titanium mesh | None | 30:70 ratio of autogenous bone graft and equine, enzyme- deantigenic collagen-preserving bone substitute |
Implants perfectly functional, bone width stable over time, heterologous biomaterial were biocompatible and undergoing advanced remodeling and replacement with newly formed bone | 24 months |
| Knöfler W [64] | Int J Implant Dent | 2016 | Titanium mesh | None | Graft materials (58.2%), membranes (36.6%), deproteinized bovine bone mineral (53%), autogenous bone particles (32.5%), native collagen membrane (74%) | Survival: 95.5%, significantly in augmented sites (p = 0.0025); best results for bone condensing method followed by lateral augmentation |
20.2 years |
| Zita Gomes R [65] | Biomed Res Int | 2016 | Titanium mesh | Edema (48%), discomfort (40%), Ti mesh exposure (24%), graft loss in 2 cases (partial and complete + 1 failure) | Bio-Oss | Horizontal bone gain: 3.67 mm (±0.89) ISR: 97.5% Peri-implant marginal bone loss: 0.43 mm (±0.15) |
1 year |
| Ahmed M [66] | Int J Oral Maxillofac Surg | 2017 | Titanium mesh | None | - | Evidence of new bone formation in both groups | 6 months |
| Cucchi A [67] | Clin Implant Dent Relat Res | 2017 | Titanium mesh | - | Both GBR approaches: similar results regarding complications, vertical bone gain, and implant stability |
3 years | |
| Jegham H [68] | J Stomatol Oral Maxillofac Surg | 2017 | Titanium mesh | Mesh exposure visible with a circular flap dehiscence at follow-up (did not affect the successful regenerative outcomes) | Autogenous bone graft mixed with a xenograft | After mesh removal from grafted defects, space was completely filled with new hard tissue covered by a thin layer of soft tissue | 4 months |
| Scarano A [69] | Oral Implantol (Rome) | 2017 | Titanium mesh | Mesh exposure in 1 of 3 P | Bone chips with resorbable membrane | Significant increase in alveolar width or height No residual bone defect observed |
12.5 years |
| Alagl AS [70] | J Int Med Res | 2018 | Titanium mesh | None | Alloplast material mixed with a nano-bone graft | Newly formed ridge dimensions: 6 H and 10 mm V (original defect: 9 mm V) Complete filling of the defect, implant success |
12 months |
| Ciocca L [71] | J Oral Implantol | 2018 | Titanium mesh | Mesh premature exposure (within 4 to 6 weeks) in 3 cases Delayed exposure (after 4 to 6 weeks) in 3 other cases Morbidity of mesh exposure (66%) |
Particulate bone grafts, autogenous bone graft and inorganic bovine bone in 1:1 ratio | Mean mandibular bone augmentation: 3.83 mm Maxillabone augmentation: 3.95 mm |
6–8 months |
| Inoue K [72] | Implant dentistry | 2018 | Titanium mesh | None | Xenograft (Bio-Oss) | - | 6 months |
| Lorenz J [73] | J Oral Implantol | 2018 | Titanium mesh | - | Xenogeneic bone substitute (BO) with platelet-rich fibrin (PRF) | Complete rehabilitation and restoration of the patient’s oral function | 16 months |
| Zhou M [74] | J Oral Implantol | 2018 | Titanium mesh | Infected graft in anterior mandible (tissue dehiscence); dehiscence 14 days after bone augmentation |
Biocoral autologous bone | Total horizontal bone gain was 4.2 ± 0.5 mm | 3 years |
| Cucchi A [75] | J Oral Implantol | 2019 | Titanium mesh | - | 50:50 mixture of autogenous bone graft and xenograft | - | 12 months |
| Di Stefano DA [59] | Dent J (Basel) | 2019 | Titanium mesh | - | Mixture of autogenous bone graft and equine-derived bone | Graft allowed effective bone formation (newly formed bone, residual Biomaterial and medullar spaces: 39%, 10%, and 51% of core volume) |
6.5 years |
| Hartmann A [76] | Implant Dent | 2019 | Titanium mesh | Exposure (26) | Advanced and injectable platelet-rich fibrin (A- and I-PRF), resorbable membranes, autogenous bone graft, and Bio-Oss | - | 12 months |
| Mounir M [77] | Clin Implant Dent Relat Res | 2019 | Titanium mesh | ||||
| Tallarico M [78] | Materials (Basel) | 2019 | Titanium mesh | Exposure after 1 month (1) | Xenograft/platelet-rich fibrin (PRF) | The mean bone gain was 5.06 ± 1.13 mm | 18 months |
| Zhang T [79] | Clin Implant Dent Relat Res | 2019 | Titanium mesh | - | Xenograft | Average bone gain values were 3.61 ± 1.50 mm vertically and 3.10 ± 2.06 mm horizontally | 41 months |
| Atef M [6] | Clin Implant Dent Relat Res | 2020 | Titanium mesh | Exposure (3) | 1:1 mixture of autogenous and inorganic bovine bone | Mean bone gain of 4.0 mm for collagen group and 3.7 mm for titanium mesh group | 6 months |
| Hartmann A [80] | BMC Oral Health | 2020 | Titanium mesh | Exposure (17) | Xenograft/A®-PRF | - | 6 months |
| Maiorana C [81] | Materials (Basel, Switzerland) | 2020 | Titanium mesh for peri-implant defects | - | Xenograft | A mean horizontal bone gain of 4.95 ± 0.96 mm, and a mean horizontal thickness of the buccal plate of 3.25 ± 0.46 mm | 8 months |
| Malik R [82] | J Maxillofac Oral Surg | 2020 | Titanium mesh | - | Novabone Putty | The mean vertical height of augmented bone was 4.825 ± 1.1387 mm | 12 months |
| Tallarico M [83] | Materials (Basel, Switzerland) | 2020 | Titanium mesh | - | Xenograft | Implant successfully supported rehabilitation; no exposure or infection were documented | 12 months |
| Li L [84] | Clin Implant Dent Relat Res | 2021 | Titanium mesh | - | Xenograft | Mean gain: 636.20 ± 341.18 mm3 | 9 months |
| Chiapasco M [85] | Clin Oral Implants Res | 2021 | Titanium mesh | Exposure (11) | Autogenous bone chips and bovine bone mineral (BBM). | The mean vertical and horizontal bone gains after reconstruction were 4.78 ± 1.88 mm and 6.35 ± 2.10 mm | 18 months |
| Cucchi A [86] | Int J Oral Implantology | 2021 | Titanium mesh | - | 50:50 bone mixtures of allograft | BV/TV, MatV/TV, and StV/TV in regenerated bone were 28.8%, 8.9%, and 62.4%, respectively In group B, the values of BV/TV, MatV/TV, and StV/TV were 30.0%, 11.0%, and 59.0% |
9 months |
| Cucchi A [87] | Clin Oral Implants Res | 2021 | Titanium mesh | Exposure (test: 2; control 4) Implant failure (3) |
50:50 bone mixtures of allograft | Better results for Mesh+ group (13%) compared to group Mesh- (33%) | 12 months |
| De Santis D [88] | Medicina (Kaunas) | 2021 | Titanium mesh | - | Xenograft | An average horizontal gain of 3.6 ± 0.8 mm and a vertical gain of 5.2 ± 1.1 mm | 9 months |
| Dellavia C [89] | Clin Implant Dent Relat Res | 2021 | Titanium mesh | - | Autogenous bone graft and deproteinized bovine bone (1:1). | 35.88% new lamellar bone, 16.42% woven bone, 10.88% of osteoid matrix, 14.10% of grafted remnants, and 22.72% of medullary spaces | 9 months |
| Kadkhodazadeh M [90] | Oral Maxillofac Surg | 2021 | Titanium mesh | - | Autogenous bone, allogenic graft material, and acellular dermal matrix | The mean marginal bone loss and bone gain were 4.4 ± 1.2 mm and 2.9 ± 0.9 mm | 60 months |
| Lee SR [13] | Materials (Basel, Switzerland) | 2021 | Titanium mesh | Exposure (test: 1; control: 1) | Xenograft (Bio-Oss) | The mean HG rate was 84.25% ± 14.19% in the CCM group and 82.56% ± 13.04% in the NCCM group | 6 months |
| Li S [91] | Int J Oral Sci | 2021 | Titanium mesh | Exposure (titanium mesh: 10%) | Autogenous bone graft and xenograft (Bio-Oss) | The percentage of resorption after 6 months of healing with resorbable membrane coverage reached 37.5%; however, it was only 23.4% with the titanium mesh | 12 months |
| Maiorana C [92] | J Contemp Dent Pract | 2021 | Titanium mesh | Exposure (2) | 1:1 ratio of autogenous bone to deproteinized bovine bone | Mean vertical bone gain of 4.2 and 1.5 mm was achieved in d-PM and TM groups | 8 months |
| Wang X [93] | Biomed Res Int | 2021 | Titanium mesh | Exposure (1) | Xenograft/CGF | The thickness of the labial bone was 3.01 mm (±0.23), 2.96 mm (±0.21), 2.93 mm (±0.19), and 2.92 mm (±0.16) at the time of the second surgery, and 6 months, 1 year, and 2 years after the surgery | 24 months |
| Yoon JH [94] | Maxillofac Plast Reconstr Surg | 2021 | Titanium mesh | Fistula | Buccal fat pad | - | 12 months |
| Bertran Faus A [95] | Materials (Basel) | 2022 | Titanium mesh | None | Autogenous bone graft and xenograft mix in 70:30 ratio | Width: 1.84 and 1.92 mm; height: 3.78 mm | 6 months |
| Boogaard MJ [20] | Compend Contin Educ Dent | 2022 | Titanium mesh | Exposure | - | Case 1: vertical gain—4.1 mm, width gain—8.7 mm; Case 2: vertical gain—6.7 mm, width gain—10.8 mm | 5.5 months |
| Del Barrio RAL [96] | J Oral Implantol | 2022 | Titanium mesh | None | Recombinant human bone morphogenetic protein-2, deproteinized bovine bone mineral | These techniques were shown to be effective after 3 years of follow-up | 3 years |
| Gelețu GL [97] | Medicina (Kaunas) | 2022 | Titanium mesh | None | Allograft bone substitute granules | Length: 11.63 mm; height: 10.34 mm | 6 months |
| Hartmann A [98] | Clin Oral Implants Res | 2022 | Titanium mesh | Pain, suppuration, BOP, implant bone resorption | Autogenous bone and xenograft (Bio-Oss) | MBL: 0.13 ± 1.84 mm (mesial); −0.13 ± 1.73 mm (distal) | 5 years |
| Levine RA [99] | Int J Periodontics Restorative Dent | 2022 | Titanium mesh | 22% minor exposure | Allograft, cellular allograft, bovine xenograft/recombinant human platelet-derived growth factor, autogenous platelet-rich growth factor, and recombinant human bone morphogenetic protein-2 | Mean horizontal gain: 4.7 ± 1.6 mm | 8 months |
| Lim J [100] | Materials (Basel) | 2022 | Titanium mesh | None | Inorganic bovine bone materials (Bio-Oss) and A-Oss xenograft (Osstem, Seoul, Korea), | Grafted volume: Bioss group—1.70 ± 0.50 cc; Bio-Oss group—1.94 ± 0.26 cc | 1 year |
| Majewski P [101] | Int J Periodontics Restorative Dent | 2022 | Titanium mesh | 50% minor exposure | Xenograft/collagen membrane | Horizontal gain: 2 mm; vertical gain: 2.75 mm | 6 months |
| Müller J [102] | Case Rep Dent | 2022 | Titanium mesh | None | Xenograft (Bioss) | Volume gain: 1.3–1.4 cm3 | 6 months |
| Poomprakobsri K [103] | J Oral Implantol | 2022 | Titanium mesh | Cumulative exposure rate: 36.9% Resorbable barrier ER: 23.3% Titanium mesh ER: 68.9%; Non-resorbable ER: 72.7% |
Xenograft | Grafted bone dimensional loss with barrier exposure (58.3%) and no barrier exposure (44.1%) | 6 months |
| Yang W [104] | BMC Oral Health | 2022 | Titanium mesh | Exposure: 1 minor, 1 major | Autogenous bone graft/deproteinized bovine bone mineral/iPRF | Mean deviation from planned GBR: −0.26 ± 0.35 mm | - |
| Abaza AWAAB [3] | Int J Oral Maxillofac Implants | 2023 | Titanium mesh | Control: dehiscence and infection (1) Test: premature/delayed exposure (4) |
Autogenous bone graft and inorganic bovine bone graft mix at 50:50 ratio | Bone width in Control group: 7.3 ± 0.9 mm; bone width in Test group: 7.0 ± 0.9 mm | 6 months |
| Attia R [105] | Int J Periodontics Restorative Dent | 2023 | Titanium mesh | Test: no exposure Control: 83% exposure |
100% Xenograft biomaterial | Lingual flap advancement in Control group: 3.9 ± 1.1 mm; Test group: 14.4 ± 3.8 mm Buccal flap advancement in Control group: 15.8 ± 2.1 mm; Test group: 10.5 ± 1.4 mm |
9 months |
| Bahaa S [106] | Int J Oral Maxillofac Surg | 2023 | Titanium mesh | None | Alloplastic bone | CALF group: 4.12 ± 1.37 mm; PRI group: 2.60 ± 1.36 mm; DFI group: 3.88 ± 1.70 mm; MPRI group: 3.44 ± 1.30 mm | 6 months |
| Chen D [21] | Clin Implant Dent Relat Res | 2023 | Titanium mesh | 100% xenograft biomaterial | No significant difference in 3D surgical positioning between the two groups | - | |
| Kurtiş B [22] | J Oral Implantol | 2023 | Titanium mesh | None | Autogenous bone graft, deproteinized bovine bone mineral, injectable platelet-rich fibrin/collagen membrane | Successfully implant supported rehabilitation; no exposure or infection were documented | 6 months |
| Nan X [107] | Clin Oral Implants Res | 2023 | Titanium mesh | Exposure rate: 32.8% | 100% xenograft biomaterial | Width: 5.22 ± 3.19 mm Height: 5.01 ± 2.83 mm Volume bone gain: 588.91 ± 361.23 mm3 |
6 months |
| Onică N | Healthcare (Basel) | 2023 | Titanium mesh | None | Xenograft allograft and an autograft/collagen membrane mix | Implant successfully supported rehabilitation; no exposure or infection were documented | 6 months |
| Onodera K [108] | J Clin Med | 2023 | Titanium mesh/neoplasm resection | Chronic pus discharge Exposure |
Autogenous particulate cancellous bone and marrow graft | Augmented length: 3.21 ± 4.94 (SD) mm Marginal bone augmentation length: −0.15 ± 0.37 mm Segmental defects length: 4.89 ± 5.34 mm |
- |
| Songhang Li [109] | Clin Implant Dent Relat Res | 2023 | Titanium mesh | None | Deproteinized bovine bone mineral (Bio-Oss) mixed with autogenous bone | - | 6 months |
| Wen SC [110] | Int J Periodontics Restorative Dent | 2023 | Titanium mesh | None | 50%/50% mixture of autograft/bovine xenograft + collagen membrane | 8.0 ± 1.0 mm horizontal bone gain 3.0 ± 0.0 mm vertical bone gain Histomorphometry: 42.8% new vital bone, 18.8% residual bone graft particles, 38.4% bone marrow |
8 months |
| Zhang G [111] | J Esthet Restor Dent | 2023 | Titanium mesh | None | 100% xenograft biomaterial | Vertical bone gain: 4.16 mm Horizontal gain: 7.48 mm |
6 months |
3.3. RoB Findings
The summary of the RoB assessment results is presented in Figure 2. According to the Cochrane Collaboration, most of the studies were considered to have a low risk of bias. According to the selection bias assessment, the findings were 71.4%lr and 28.6%hr regarding random sequence generation. The performance bias and detection bias analyses reported 28.6%lr and 71.4%ur for these studies. The attrition bias was 28.6%ur and 71.4%lr (Figure 3). A value of 100%lr was reported for the allocation concealment, selective reporting, and other biases.
Figure 2.
Risk of bias graph: review authors’ judgements about each risk of bias item, presented as percentages for all included studies.
Figure 3.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study [green light (+): low RoB; yellow light (?) uncertain RoB; red light (−): high RoB] [6,67,87,105,106,109].
3.4. Meta-Analysis Assessment
3.4.1. Titanium Mesh vs. Membrane GBR: Site Exposure
This assessment included four articles for a total of 130 participants (range: 20–40). The estimated effect was 2.56 [0.91; 7.20]. The heterogeneity test reported a Chi2 value of 0.91 and I2 of 0%. No significant differences between the study groups were reported in terms of exposure of the site during the healing period (p = 0.08) (Figure 4). The exposure ratio of the titanium mesh and GBR groups were, respectively, 21.53% and 9.23%.
Figure 4.
Forest plot of comparison of exposure outcome for mesh group vs. GBR group [6,67,87,109].
3.4.2. Titanium Mesh vs. Membrane GBR: Vertical Bone Gain (VBG)
This assessment included four articles for a total of 130 participants (range: 20–40). The estimated effect was −0.12 [−0.81; 0.58]. The heterogeneity test reported a Chi2 value of 6.43 and I2 of 53%. No significant differences between the study groups were reported in terms of the VBG (p = 0.74) (Figure 5). The mean VBG of the titanium mesh and GBR groups were, respectively, 4.22 ± 1.68 and 4.42 ± 1.18.
Figure 5.
Forest plot of comparison of vertical bone gain outcome for mesh group vs. GBR group [6,67,87,109].
3.4.3. Coronally Advanced Lingual Flap Site Exposure
This assessment included two articles for a total of 54 participants (range: 14–40). The estimated effect was 0.10 [0.01; 0.94]. The heterogeneity test reported a Chi2 value of 0.66 and I2 of 0%. Significant differences between the study groups were reported in terms of the exposure of the site during the healing period (p = 0.04) (Figure 6). The exposure ratios of the coronally advanced lingual flap and control groups were, respectively, 0% and 43.2%.
Figure 6.
Forest plot of comparison of lingual flap release exposure [6,106].
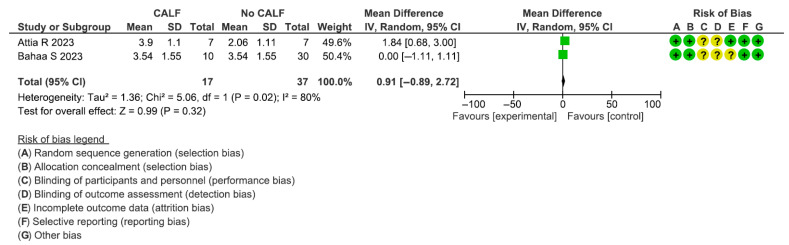
3.4.4. Coronally Advanced Lingual Flap Vertical Bone Gain (VBG)
This assessment included two articles for a total of 54 participants (range: 14–40). The estimated effect was 0.91 [−0.89; 2.72]. The heterogeneity test reported a Chi2 value of 5.06 and I2 of 80%. No significant differences between the study groups were reported in terms of the VBG (p = 0.32) (Figure 7). The VBG of the coronally advanced lingual flap and control groups were, respectively, 3.68 ± 1.36 and 3.26 ± 1.47.
Figure 7.
Forest plot of comparison of vertical bone gain (VBG) [105,106].
4. Discussion
The use of titanium mesh in the reconstruction of localized bone defects has been used with high reliability and very low exposure and complication rates [112]. Titanium mesh has been indicated for a wide range of clinical defects including peri-implant bone defects, maxillary atrophy, alveolar sockets, and periodontal defects, and for other therapeutic applications [90]. The literature documents its use in a small number of cases for more extensive defects that originate from neoplastic pathological processes, such as odontogenic keratocyst processes, and trauma treated with complete ostectomy, hemibulectomies, and completely disarticulated resections of mandible and mandibular rami [27,113]. There are documented cases of titanium mesh use in non-grafted sinus floor elevation [114]. Titanium meshes are high-performance devices with high biocompatibility; their the barrier effect can guide the healing processes in the absence of immune responses during healing [102]. The grids can be morphologically adapted to the defect which makes them highly specific and customizable; such customization can be achieved using laser sintering or CAD/CAM [102]. Titanium grids can be stabilized with microscrews on the sides of the membrane itself and can be equipped with holes that allow for a greater blood supply to the defect, bringing oxygen, nutrients, and immune cells into the defect, which are essential to ensure the success of osteogenesis. Studies have confirmed that macroporosity has effects on bone regeneration by ensuring a sufficient blood supply to the defect, stimulating osteogenesis due to the presence of the holes. It has been observed that titanium meshes do not interfere with blood flow [21]. In addition, the presence of the mesh, compared with the presence of resorbable membranes alone, ensures that the treatment is not compromised; thus, they are considered reliable for promoting new bone formation. The rigidity of the titanium mesh ensures stability and prevents the collapse of the membrane itself within the defect, a situation that is possible with the use of resorbable membranes alone [25,56,115]. In some cases of combination regenerative and implant therapies, the implants were placed concurrently with the titanium mesh; in other cases, the placement of the titanium mesh occurred in a second surgery after 8–9 months [107]. Regenerative procedures using titanium meshes resulted in significant bone regeneration in the narrow alveolar ridges, allowing for implant placement [39]. Regenerative site exposure seems to be one of the most common early and delayed complication during the healing period. The present investigation reported no significant differences in exposure rate for titanium mesh vs. membrane GBR procedures (p = 0.06). Fewer exposures were observed with the use of e-PTFE membranes to cover the titanium meshes [15]. Due to the tight spread of the study outcome and the limited number of selected articles, this aspect deserves more study to determine the exposure outcome. A critical point of the present investigation was to analyze the wide heterogeneity in methods including the treatment site and jaw region, defect extension, simultaneous/delayed implant positioning, graft materials, and additional screws and plates used.
Following to the Cochrane review methodology, the present review performed a search in multiple electronic databases. Due to the difficulties in finding MesH term indicators for this topic, the screening was conducted considering all clinical studies and without applying filters regarding the study design methodology for the full-text evaluation, eligibility analysis, and descriptive synthesis. The statistical methods considered the applicability of sub-group comparisons. The main limit of this approach is indubitably a decrease in the study data robustness and strength that should be considered when interpreting the findings from this review. The present study considered an observation period of only about 9 months but a more extended follow-up period is necessary to evaluate the medium- and long-term effectiveness of both techniques in over to evaluate the comparative performance of mesh regeneration compared to membrane grafts.
Our opinion is that homogeneous study methodologies are necessary to improve the robustness of meta-analyses. It is necessary for review methodologies to reduce the wide range of biases associated with several variables including the surgical technique, procedure site (single/multiple edentulism), atrophy grading, mesh characteristics (including the porosity), adaptation technique, use of stabilization screws, and biomaterial used. In fact, considering the wide range of biomaterials used and the differences in methodology, a meta-analysis was not possible.
A sufficient pool of articles for a pairwise comparison was only possible for an analysis of the VBG and coronally advanced lingual flap as statistical variables based on the methodological characteristics and RoB of the considered studies. The VBG also seemed to be similar for both clinical protocols; more histological comparisons could elucidate the graft-interface differences and the new bone formation patterns between the procedures. A drastic reduction in the exposure rates was reported for the coronally advanced lingual flap method, suggesting that it could be considered a favorable approach for decreasing the incidence of complications. No effects were reported for the vertical bone gain parameters. In addition, treating large defects with a customized titanium mesh is a useful protocol and provides a predictable result, even in the case of dehiscence. Custom, pre-formed titanium mesh together with a mixture of autologous bone and a xenograft is a feasible and reliable technique for vertical bone regeneration and advanced and three-dimensional defects [95].
5. Conclusions
Due to the weak robustness of the study data, the limitations of the present review, and the strength of the analytic findings, no definitive conclusions could be made but this topic is worthy of further investigation in the future. The research outcome seems to suggest that bone regeneration of more extensive defects using titanium meshes represents a useful bone regeneration technique, which, despite being performed with different methods using different combinations of membranes and/or bone grafts of different types, and its possible complications, was found to not compromise regenerative techniques. In the present investigation, no significant differences in bone exposure and vertical bone gain were observed when comparing the technique with membrane bone regeneration. The physical and morphological characteristics of the titanium meshes, which can also be customized to the conformation of the defect, guarantee the immobilization and stability of the defect and thus will guide the regeneration and, when present, the optimal integration of the biomaterial. The management and the surgical passivity of the flaps seems to minimize the risk of exposure, with a significant reduction in the complication incidence.
Author Contributions
Conceptualization, A.S. and F.L.; methodology, F.L., I.A., S.A.G. and S.R.T.; software, F.L.; validation, A.S., F.L., S.A.G., S.R.T. and I.A.; formal analysis, F.L.; investigation, F.L., I.A., S.A.G. and S.R.T.; data curation, F.L., I.A., S.A.G. and S.R.T.; writing—original draft preparation, F.L.; writing—review and editing, F.L.; visualization, F.L.; supervision, F.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All experimental data that support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ali S.A., Karthigeyan S., Deivanai M., Kumar A. Implant Rehabilitation for Atrophic Maxilla: A Review. J. Indian Prosthodont. Soc. 2014;14:196–207. doi: 10.1007/s13191-014-0360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tumedei M., Mijiritsky E., Mourão C.F., Piattelli A., Degidi M., Mangano C., Iezzi G. Histological and Biological Response to Different Types of Biomaterials: A Narrative Single Research Center Experience over Three Decades. Int. J. Environ. Res. Public Health. 2022;19:7942. doi: 10.3390/ijerph19137942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abaza A.W.A.A.B., Abbas W.M., Khalik D.M.A., El Din N.H.K. Horizontal Ridge Augmentation of the Atrophic Maxilla Using Pericardium Membrane Versus Titanium Mesh: A Clinical and Histologic Randomized Comparative Study. Int. J. Oral Maxillofac. Implant. 2023;38:451–461. doi: 10.11607/jomi.9715. [DOI] [PubMed] [Google Scholar]
- 4.Santamaría J., García A.M., de Vicente J.C., Landa S., López-Arranz J.S. Bone Regeneration after Radicular Cyst Removal with and without Guided Bone Regeneration. Int. J. Oral Maxillofac. Surg. 1998;27:118–120. doi: 10.1016/S0901-5027(98)80308-1. [DOI] [PubMed] [Google Scholar]
- 5.Aghazadeh A., Persson R.G., Renvert S. Impact of Bone Defect Morphology on the Outcome of Reconstructive Treatment of Peri-Implantitis. Int. J. Implant. Dent. 2020;6:33. doi: 10.1186/s40729-020-00219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atef M., Tarek A., Shaheen M., Alarawi R.M., Askar N. Horizontal Ridge Augmentation Using Native Collagen Membrane vs Titanium Mesh in Atrophic Maxillary Ridges: Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2020;22:156–166. doi: 10.1111/cid.12892. [DOI] [PubMed] [Google Scholar]
- 7.Chavda S., Levin L. Human Studies of Vertical and Horizontal Alveolar Ridge Augmentation Comparing Different Types of Bone Graft Materials: A Systematic Review. J. Oral Implantol. 2018;44:74–84. doi: 10.1563/aaid-joi-D-17-00053. [DOI] [PubMed] [Google Scholar]
- 8.Scarano A., Carinci F., Assenza B., Piattelli M., Murmura G., Piattelli A. Vertical Ridge Augmentation of Atrophic Posterior Mandible Using an Inlay Technique with a Xenograft without Miniscrews and Miniplates: Case Series. Clin. Oral Implant. Res. 2011;22:1125–1130. doi: 10.1111/j.1600-0501.2010.02083.x. [DOI] [PubMed] [Google Scholar]
- 9.Iezzi G., Degidi M., Piattelli A., Mangano C., Scarano A., Shibli J.A., Perrotti V. Comparative Histological Results of Different Biomaterials Used in Sinus Augmentation Procedures: A Human Study at 6 Months. Clin. Oral Implant. Res. 2012;23:1369–1376. doi: 10.1111/j.1600-0501.2011.02308.x. [DOI] [PubMed] [Google Scholar]
- 10.de Azambuja Carvalho P.H., Dos Santos Trento G., Moura L.B., Cunha G., Gabrielli M.A.C., Pereira-Filho V.A. Horizontal Ridge Augmentation Using Xenogenous Bone Graft-Systematic Review. Oral Maxillofac. Surg. 2019;23:271–279. doi: 10.1007/s10006-019-00777-y. [DOI] [PubMed] [Google Scholar]
- 11.Briguglio F., Falcomatà D., Marconcini S., Fiorillo L., Briguglio R., Farronato D. The Use of Titanium Mesh in Guided Bone Regeneration: A Systematic Review. Int. J. Dent. 2019;2019:9065423. doi: 10.1155/2019/9065423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graziano A., d’Aquino R., Angelis M.G.C.-D., De Francesco F., Giordano A., Laino G., Piattelli A., Traini T., De Rosa A., Papaccio G. Scaffold’s Surface Geometry Significantly Affects Human Stem Cell Bone Tissue Engineering. J. Cell. Physiol. 2008;214:166–172. doi: 10.1002/jcp.21175. [DOI] [PubMed] [Google Scholar]
- 13.Lee S.-R., Jang T.-S., Seo C.-S., Choi I.-O., Lee W.-P. Hard Tissue Volume Stability Effect beyond the Bony Envelope of a Three-Dimensional Preformed Titanium Mesh with Two Different Collagen Barrier Membranes on Peri-Implant Dehiscence Defects in the Anterior Maxilla: A Randomized Clinical Trial. Materials. 2021;14:5618. doi: 10.3390/ma14195618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Hezaimi K., Iezzi G., Rudek I., Al-Daafas A., Al-Hamdan K., Al-Rasheed A., Javed F., Piattelli A., Wang H.-L. Histomorphometric Analysis of Bone Regeneration Using a Dual Layer of Membranes (dPTFE Placed over Collagen) in Fresh Extraction Sites: A Canine Model. J. Oral Implant. 2015;41:188–195. doi: 10.1563/AAID-JOI-D-13-00027. [DOI] [PubMed] [Google Scholar]
- 15.Simion M., Baldoni M., Rossi P., Zaffe D. A Comparative Study of the Effectiveness of E-PTFE Membranes with and without Early Exposure during the Healing Period. Int. J. Periodontics Restor. Dent. 1994;14:166–180. [PubMed] [Google Scholar]
- 16.Colangelo P., Piattelli A., Barrucci S., Trisi P., Formisano G., Caiazza S. Bone Regeneration Guided by Resorbable Collagen Membranes in Rabbits: A Pilot Study. Implant. Dent. 1993;2:101–105. doi: 10.1097/00008505-199305000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Ren Y., Fan L., Alkildani S., Liu L., Emmert S., Najman S., Rimashevskiy D., Schnettler R., Jung O., Xiong X., et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022;23:14987. doi: 10.3390/ijms232314987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higuchi M., Moroi A., Yoshizawa K., Kosaka A., Ikawa H., Iguchi R., Saida Y., Hotta A., Tsutsui T., Ueki K. Comparison between Various Densities of Pore Titanium Meshes and E-Polytetrafluoroethylene (ePTFE) Membrane Regarding Bone Regeneration Induced by Low Intensity Pulsed Ultrasound (LIPUS) in Rabbit Nasal Bone. J. Craniomaxillofac. Surg. 2016;44:1152–1161. doi: 10.1016/j.jcms.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Aerts E., Li J., Van Steenbergen M.J., Degrande T., Jansen J.A., Walboomers X.F. Porous Titanium Fiber Mesh with Tailored Elasticity and Its Effect on Stromal Cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020;108:2180–2191. doi: 10.1002/jbm.b.34556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boogaard M.J., Santoro F., Romanos G.E. Mesh Ridge Augmentation Using CAD/CAM Technology for Design and Printing: Two Case Reports. Compend. Contin. Educ. Dent. 2022;43:654–663. [PubMed] [Google Scholar]
- 21.Chen D., Zheng L., Wang C., Huang Y., Huang H., Apicella A., Hu G., Wang L., Fan Y. Evaluation of Surgical Placement Accuracy of Customized CAD/CAM Titanium Mesh Using Screws-Position-Guided Template: A Retrospective Comparative Study. Clin. Implant. Dent. Relat. Res. 2023;25:519–531. doi: 10.1111/cid.13205. [DOI] [PubMed] [Google Scholar]
- 22.Kurtiş B., Şahin S., Gürbüz S., Yurduseven S., Altay C., Kurtiş B., Ayyıldız S., Barış E. Vertical Bone Augmentation with Customized CAD/CAM Titanium Mesh for Severe Alveolar Ridge Defect in the Posterior Mandible: A Case Letter. J. Oral Implant. 2023;49:147–156. doi: 10.1563/aaid-joi-D-22-00094. [DOI] [PubMed] [Google Scholar]
- 23.Gu C., Xu L., Shi A., Guo L., Chen H., Qin H. Titanium Mesh Exposure in Guided Bone Regeneration Procedures: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2022;37:e29–e40. doi: 10.11607/jomi.9098. [DOI] [PubMed] [Google Scholar]
- 24.Boyne P.J. The Restoration of Resected Mandibles in Children without the Use of Bone Grafts. Head Neck Surg. 1983;6:626–631. doi: 10.1002/hed.2890060203. [DOI] [PubMed] [Google Scholar]
- 25.Malchiodi L., Scarano A., Quaranta M., Piattelli A. Rigid Fixation by Means of Titanium Mesh in Edentulous Ridge Expansion for Horizontal Ridge Augmentation in the Maxilla. Int. J. Oral Maxillofac. Implant. 1998;13:701–705. [PubMed] [Google Scholar]
- 26.von Arx T., Kurt B. Implant Placement and Simultaneous Peri-Implant Bone Grafting Using a Micro Titanium Mesh for Graft Stabilization. Int. J. Periodontics Restor. Dent. 1998;18:117–127. [PubMed] [Google Scholar]
- 27.von Arx T., Kurt B. Implant Placement and Simultaneous Ridge Augmentation Using Autogenous Bone and a Micro Titanium Mesh: A Prospective Clinical Study with 20 Implants. Clin. Oral Implant. Res. 1999;10:24–33. doi: 10.1034/j.1600-0501.1999.100104.x. [DOI] [PubMed] [Google Scholar]
- 28.Sumi Y., Miyaishi O., Tohnai I., Ueda M. Alveolar Ridge Augmentation with Titanium Mesh and Autogenous Bone. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000;89:268–270. doi: 10.1016/S1079-2104(00)70087-4. [DOI] [PubMed] [Google Scholar]
- 29.Klug C.N., Millesi-Schobel G.A., Millesi W., Watzinger F., Ewers R. Preprosthetic Vertical Distraction Osteogenesis of the Mandible Using an L-Shaped Osteotomy and Titanium Membranes for Guided Bone Regeneration. J. Oral Maxillofac. Surg. 2001;59:1302–1308. doi: 10.1053/joms.2001.27520. discussion 1309–1310. [DOI] [PubMed] [Google Scholar]
- 30.Maiorana C., Santoro F., Rabagliati M., Salina S. Evaluation of the Use of Iliac Cancellous Bone and Anorganic Bovine Bone in the Reconstruction of the Atrophic Maxilla with Titanium Mesh: A Clinical and Histologic Investigation. Int. J. Oral Maxillofac. Implant. 2001;16:427–432. [PubMed] [Google Scholar]
- 31.Artzi Z., Dayan D., Alpern Y., Nemcovsky C.E. Vertical Ridge Augmentation Using Xenogenic Material Supported by a Configured Titanium Mesh: Clinicohistopathologic and Histochemical Study. Int. J. Oral Maxillofac. Implant. 2003;18:440–446. [PubMed] [Google Scholar]
- 32.Degidi M., Scarano A., Piattelli A. Regeneration of the Alveolar Crest Using Titanium Micromesh with Autologous Bone and a Resorbable Membrane. J. Oral Implantol. 2003;29:86–90. doi: 10.1563/1548-1336(2003)029<0086:ROTACU>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Proussaefs P., Lozada J., Kleinman A., Rohrer M.D., McMillan P.J. The Use of Titanium Mesh in Conjunction with Autogenous Bone Graft and Inorganic Bovine Bone Mineral (Bio-Oss) for Localized Alveolar Ridge Augmentation: A Human Study. Int. J. Periodontics Restor. Dent. 2003;23:185–195. [PubMed] [Google Scholar]
- 34.Roccuzzo M., Ramieri G., Spada M.C., Bianchi S.D., Berrone S. Vertical Alveolar Ridge Augmentation by Means of a Titanium Mesh and Autogenous Bone Grafts. Clin. Oral Implant. Res. 2004;15:73–81. doi: 10.1111/j.1600-0501.2004.00998.x. [DOI] [PubMed] [Google Scholar]
- 35.Proussaefs P., Lozada J. Use of Titanium Mesh for Staged Localized Alveolar Ridge Augmentation: Clinical and Histologic-Histomorphometric Evaluation. J. Oral Implant. 2006;32:237–247. doi: 10.1563/1548-1336(2006)32[237:UOTMFS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Kfir E., Kfir V., Kaluski E. Immediate Bone Augmentation after Infected Tooth Extraction Using Titanium Membranes. J. Oral Implant. 2007;33:133–138. doi: 10.1563/1548-1336(2007)33[133:IBAAIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Longoni S., Sartori M., Apruzzese D., Baldoni M. Preliminary Clinical and Histologic Evaluation of a Bilateral 3-Dimensional Reconstruction in an Atrophic Mandible: A Case Report. Int. J. Oral Maxillofac. Implant. 2007;22:478–483. [PubMed] [Google Scholar]
- 38.Roccuzzo M., Ramieri G., Bunino M., Berrone S. Autogenous Bone Graft Alone or Associated with Titanium Mesh for Vertical Alveolar Ridge Augmentation: A Controlled Clinical Trial. Clin. Oral. Implant. Res. 2007;18:286–294. doi: 10.1111/j.1600-0501.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 39.Aikawa T., Iida S., Senoo H., Hori K., Namikawa M., Okura M., Ono T. Widening a Narrow Posterior Mandibular Alveolus Following Extirpation of a Large Cyst: A Case Treated with a Titanium Mesh-Plate Type Distractor. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008;106:e1–e7. doi: 10.1016/j.tripleo.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Pieri F., Corinaldesi G., Fini M., Aldini N.N., Giardino R., Marchetti C. Alveolar Ridge Augmentation with Titanium Mesh and a Combination of Autogenous Bone and Anorganic Bovine Bone: A 2-Year Prospective Study. J. Periodontol. 2008;79:2093–2103. doi: 10.1902/jop.2008.080061. [DOI] [PubMed] [Google Scholar]
- 41.Corinaldesi G., Pieri F., Sapigni L., Marchetti C. Evaluation of Survival and Success Rates of Dental Implants Placed at the Time of or after Alveolar Ridge Augmentation with an Autogenous Mandibular Bone Graft and Titanium Mesh: A 3 to 8-Year Retrospective Study. Int. J. Oral Maxillofac. Implant. 2009;24:1119–1128. [PubMed] [Google Scholar]
- 42.Torres J., Tamimi F., Alkhraisat M.H., Manchón A., Linares R., Prados-Frutos J.C., Hernández G., López Cabarcos E. Platelet-Rich Plasma May Prevent Titanium-Mesh Exposure in Alveolar Ridge Augmentation with Anorganic Bovine Bone. J. Clin. Periodontol. 2010;37:943–951. doi: 10.1111/j.1600-051X.2010.01615.x. [DOI] [PubMed] [Google Scholar]
- 43.Ciocca L., Fantini M., De Crescenzio F., Corinaldesi G., Scotti R. Direct Metal Laser Sintering (DMLS) of a Customized Titanium Mesh for Prosthetically Guided Bone Regeneration of Atrophic Maxillary Arches. Med. Biol. Eng. Comput. 2011;49:1347–1352. doi: 10.1007/s11517-011-0813-4. [DOI] [PubMed] [Google Scholar]
- 44.Misch C.M. Bone Augmentation of the Atrophic Posterior Mandible for Dental Implants Using rhBMP-2 and Titanium Mesh: Clinical Technique and Early Results. Int. J. Periodontics. Restor. Dent. 2011;31:581–589. [PubMed] [Google Scholar]
- 45.Cicciù M., Herford A.S., Stoffella E., Cervino G., Cicciù D. Protein-Signaled Guided Bone Regeneration Using Titanium Mesh and Rh-BMP2 in Oral Surgery: A Case Report Involving Left Mandibular Reconstruction after Tumor Resection. Open. Dent. J. 2012;6:51–55. doi: 10.2174/1874210601206010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Her S., Kang T., Fien M.J. Titanium Mesh as an Alternative to a Membrane for Ridge Augmentation. J. Oral. Maxillofac. Surg. 2012;70:803–810. doi: 10.1016/j.joms.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 47.Miyamoto I., Funaki K., Yamauchi K., Kodama T., Takahashi T. Alveolar Ridge Reconstruction with Titanium Mesh and Autogenous Particulate Bone Graft: Computed Tomography-Based Evaluations of Augmented Bone Quality and Quantity. Clin. Implant. Dent. Relat. Res. 2012;14:304–311. doi: 10.1111/j.1708-8208.2009.00257.x. [DOI] [PubMed] [Google Scholar]
- 48.Ciocca L., Fantini M., De Crescenzio F., Corinaldesi G., Scotti R. CAD-CAM Prosthetically Guided Bone Regeneration Using Preformed Titanium Mesh for the Reconstruction of Atrophic Maxillary Arches. Comput. Methods Biomech. Biomed. Engin. 2013;16:26–32. doi: 10.1080/10255842.2011.601279. [DOI] [PubMed] [Google Scholar]
- 49.Funato A., Ishikawa T., Kitajima H., Yamada M., Moroi H. A Novel Combined Surgical Approach to Vertical Alveolar Ridge Augmentation with Titanium Mesh, Resorbable Membrane, and rhPDGF-BB: A Retrospective Consecutive Case Series. Int. J. Periodontics. Restor. Dent. 2013;33:437–445. doi: 10.11607/prd.1460. [DOI] [PubMed] [Google Scholar]
- 50.Atef M., Hakam M.M., ElFaramawey M.I., Abou-ElFetouh A., Ekram M. Nongrafted Sinus Floor Elevation with a Space-Maintaining Titanium Mesh: Case-Series Study on Four Patients. Clin. Implant. Dent. Relat. Res. 2014;16:893–903. doi: 10.1111/cid.12064. [DOI] [PubMed] [Google Scholar]
- 51.Butura C.C., Galindo D.F. Implant Placement in Alveolar Composite Defects Regenerated with rhBMP-2, Anorganic Bovine Bone, and Titanium Mesh: A Report of Eight Reconstructed Sites. Int. J. Oral Maxillofac. Implant. 2014;29:e139–e146. doi: 10.11607/jomi.te53. [DOI] [PubMed] [Google Scholar]
- 52.Jung G.-U., Jeon J.-Y., Hwang K.-G., Park C.-J. Preliminary Evaluation of a Three-Dimensional, Customized, and Preformed Titanium Mesh in Peri-Implant Alveolar Bone Regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2014;40:181–187. doi: 10.5125/jkaoms.2014.40.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katanec D., Granić M., Majstorović M., Trampus Z., Pandurić D.G. Use of Recombinant Human Bone Morphogenetic Protein (rhBMP2) in Bilateral Alveolar Ridge Augmentation: Case Report. Coll. Antropol. 2014;38:325–330. [PubMed] [Google Scholar]
- 54.Levine R.A., Manji A., Faucher J., Fava P. Use of Titanium Mesh in Implant Site Development for Restorative-Driven Implant Placement: Case Report. Part 2: Surgical Protocol for Single-Tooth Esthetic Zone Sites. Compend. Contin. Educ. Dent. 2014;35:324; 326; 328; 330–333. [PubMed] [Google Scholar]
- 55.Lizio G., Corinaldesi G., Marchetti C. Alveolar Ridge Reconstruction with Titanium Mesh: A Three-Dimensional Evaluation of Factors Affecting Bone Augmentation. Int. J. Oral Maxillofac. Implant. 2014;29:1354–1363. doi: 10.11607/jomi.3417. [DOI] [PubMed] [Google Scholar]
- 56.Poli P.P., Beretta M., Cicciù M., Maiorana C. Alveolar Ridge Augmentation with Titanium Mesh. A Retrospective Clinical Study. Open. Dent. J. 2014;8:148–158. doi: 10.2174/1874210601408010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vrielinck L., Sun Y., Schepers S., Politis C., Van Slycke S., Agbaje J.O. Osseous Reconstruction Using an Occlusive Titanium Membrane Following Marginal Mandibulectomy: Proof of Principle. J. Craniofac. Surg. 2014;25:1112–1114. doi: 10.1097/SCS.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 58.De Angelis N., De Lorenzi M., Benedicenti S. Surgical Combined Approach for Alveolar Ridge Augmentation with Titanium Mesh and rhPDGF-BB: A 3-Year Clinical Case Series. Int. J. Periodontics. Restor. Dent. 2015;35:231–237. doi: 10.11607/prd.2234. [DOI] [PubMed] [Google Scholar]
- 59.Di Stefano D.A., Greco G., Gherlone E. A Preshaped Titanium Mesh for Guided Bone Regeneration with an Equine-Derived Bone Graft in a Posterior Mandibular Bone Defect: A Case Report. Dent. J. 2019;7:77. doi: 10.3390/dj7030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim Y., Leem D.H. Post Traumatic Immediate GBR: Alveolar Ridge Preservation after a Comminuted Fracture of the Anterior Maxilla. Dent. Traumatol. 2015;31:156–159. doi: 10.1111/edt.12144. [DOI] [PubMed] [Google Scholar]
- 61.Lee H.-G., Kim Y.-D. Volumetric Stability of Autogenous Bone Graft with Mandibular Body Bone: Cone-Beam Computed Tomography and Three-Dimensional Reconstruction Analysis. J. Korean Assoc. Oral Maxillofac. Surg. 2015;41:232–239. doi: 10.5125/jkaoms.2015.41.5.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sumida T., Otawa N., Kamata Y.U., Kamakura S., Mtsushita T., Kitagaki H., Mori S., Sasaki K., Fujibayashi S., Takemoto M., et al. Custom-Made Titanium Devices as Membranes for Bone Augmentation in Implant Treatment: Clinical Application and the Comparison with Conventional Titanium Mesh. J. Craniomaxillofac. Surg. 2015;43:2183–2188. doi: 10.1016/j.jcms.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 63.Misch C.M., Jensen O.T., Pikos M.A., Malmquist J.P. Vertical Bone Augmentation Using Recombinant Bone Morphogenetic Protein, Mineralized Bone Allograft, and Titanium Mesh: A Retrospective Cone Beam Computed Tomography Study. Int. J. Oral Maxillofac. Implant. 2015;30:202–207. doi: 10.11607/jomi.3977. [DOI] [PubMed] [Google Scholar]
- 64.Knöfler W., Barth T., Graul R., Krampe D. Retrospective Analysis of 10,000 Implants from Insertion up to 20 Years-Analysis of Implantations Using Augmentative Procedures. Int. J. Implant. Dent. 2016;2:25. doi: 10.1186/s40729-016-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zita Gomes R., Paraud Freixas A., Han C.-H., Bechara S., Tawil I. Alveolar Ridge Reconstruction with Titanium Meshes and Simultaneous Implant Placement: A Retrospective, Multicenter Clinical Study. Biomed. Res. Int. 2016;2016:5126838. doi: 10.1155/2016/5126838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmed M., Abu Shama A., Hamdy R.M., Ezz M. Bioresorbable versus Titanium Space-Maintaining Mesh in Maxillary Sinus Floor Elevation: A Split-Mouth Study. Int. J. Oral. Maxillofac. Surg. 2017;46:1178–1187. doi: 10.1016/j.ijom.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Cucchi A., Vignudelli E., Napolitano A., Marchetti C., Corinaldesi G. Evaluation of Complication Rates and Vertical Bone Gain after Guided Bone Regeneration with Non-Resorbable Membranes versus Titanium Meshes and Resorbable Membranes. A Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2017;19:821–832. doi: 10.1111/cid.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jegham H., Masmoudi R., Ouertani H., Blouza I., Turki S., Khattech M.B. Ridge Augmentation with Titanium Mesh: A Case Report. J. Stomatol. Oral Maxillofac. Surg. 2017;118:181–186. doi: 10.1016/j.jormas.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Scarano A. Delayed Expansion of Atrophic Mandible (Deam): A Case Report. ORL. 2017;10:190–196. doi: 10.11138/orl/2017.10.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alagl A.S., Madi M. Localized Ridge Augmentation in the Anterior Maxilla Using Titanium Mesh, an Alloplast, and a Nano-Bone Graft: A Case Report. J. Int. Med. Res. 2018;46:2001–2007. doi: 10.1177/0300060518758226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ciocca L., Lizio G., Baldissara P., Sambuco A., Scotti R., Corinaldesi G. Prosthetically CAD-CAM-Guided Bone Augmentation of Atrophic Jaws Using Customized Titanium Mesh: Preliminary Results of an Open Prospective Study. J. Oral Implant. 2018;44:131–137. doi: 10.1563/aaid-joi-D-17-00125. [DOI] [PubMed] [Google Scholar]
- 72.Inoue K., Nakajima Y., Omori M., Suwa Y., Kato-Kogoe N., Yamamoto K., Kitagaki H., Mori S., Nakano H., Ueno T. Reconstruction of the Alveolar Bone Using Bone Augmentation with Selective Laser Melting Titanium Mesh Sheet: A Report of 2 Cases. Implant. Dent. 2018;27:602–607. doi: 10.1097/ID.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 73.Lorenz J., Al-Maawi S., Sader R., Ghanaati S. Individualized Titanium Mesh Combined with Platelet-Rich Fibrin and Deproteinized Bovine Bone: A New Approach for Challenging Augmentation. J. Oral. Implant. 2018;44:345–351. doi: 10.1563/aaid-joi-D-18-00049. [DOI] [PubMed] [Google Scholar]
- 74.Zhou M., Li S.-Y., Terheyden H., Cao S.-S., Che Y.-J., Geng Y.-M. Particulate Coral Hydroxyapatite Sheltered by Titanium Mesh for Localized Alveolar Rehabilitation After Onlay Graft Failure: A Case Report. J. Oral. Implant. 2018;44:147–152. doi: 10.1563/aaid-joi-D-17-00109. [DOI] [PubMed] [Google Scholar]
- 75.Cucchi A., Giavatto M.A., Giannatiempo J., Lizio G., Corinaldesi G. Custom-Made Titanium Mesh for Maxillary Bone Augmentation with Immediate Implants and Delayed Loading. J. Oral. Implant. 2019;45:59–64. doi: 10.1563/aaid-joi-D-18-00141. [DOI] [PubMed] [Google Scholar]
- 76.Hartmann A., Hildebrandt H., Schmohl J.U., Kämmerer P.W. Evaluation of Risk Parameters in Bone Regeneration Using a Customized Titanium Mesh: Results of a Clinical Study. Implant. Dent. 2019;28:543–550. doi: 10.1097/ID.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 77.Mounir M., Shalash M., Mounir S., Nassar Y., El Khatib O. Assessment of Three Dimensional Bone Augmentation of Severely Atrophied Maxillary Alveolar Ridges Using Prebent Titanium Mesh vs Customized Poly-Ether-Ether-Ketone (PEEK) Mesh: A Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2019;21:960–967. doi: 10.1111/cid.12748. [DOI] [PubMed] [Google Scholar]
- 78.Tallarico M., Ceruso F.M., Muzzi L., Meloni S.M., Kim Y.-J., Gargari M., Martinolli M. Effect of Simultaneous Immediate Implant Placement and Guided Bone Reconstruction with Ultra-Fine Titanium Mesh Membranes on Radiographic and Clinical Parameters after 18 Months of Loading. Materials. 2019;12:1710. doi: 10.3390/ma12101710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang T., Zhang T., Cai X. The Application of a Newly Designed L-Shaped Titanium Mesh for GBR with Simultaneous Implant Placement in the Esthetic Zone: A Retrospective Case Series Study. Clin. Implant. Dent. Relat. Res. 2019;21:862–872. doi: 10.1111/cid.12726. [DOI] [PubMed] [Google Scholar]
- 80.Hartmann A., Seiler M. Minimizing Risk of Customized Titanium Mesh Exposures—A Retrospective Analysis. BMC Oral Health. 2020;20:36. doi: 10.1186/s12903-020-1023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maiorana C., Manfredini M., Beretta M., Signorino F., Bovio A., Poli P.P. Clinical and Radiographic Evaluation of Simultaneous Alveolar Ridge Augmentation by Means of Preformed Titanium Meshes at Dehiscence-Type Peri-Implant Defects: A Prospective Pilot Study. Materials. 2020;13:2389. doi: 10.3390/ma13102389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malik R., Gupta A., Bansal P., Sharma R., Sharma S. Evaluation of Alveolar Ridge Height Gained by Vertical Ridge Augmentation Using Titanium Mesh and Novabone Putty in Posterior Mandible. J. Maxillofac. Oral Surg. 2020;19:32–39. doi: 10.1007/s12663-019-01250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tallarico M., Park C.-J., Lumbau A.I., Annucci M., Baldoni E., Koshovari A., Meloni S.M. Customized 3D-Printed Titanium Mesh Developed to Regenerate a Complex Bone Defect in the Aesthetic Zone: A Case Report Approached with a Fully Digital Workflow. Materials. 2020;13:3874. doi: 10.3390/ma13173874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L., Wang C., Li X., Fu G., Chen D., Huang Y. Research on the Dimensional Accuracy of Customized Bone Augmentation Combined with 3D-Printing Individualized Titanium Mesh: A Retrospective Case Series Study. Clin. Implant. Dent. Relat. Res. 2021;23:5–18. doi: 10.1111/cid.12966. [DOI] [PubMed] [Google Scholar]
- 85.Chiapasco M., Casentini P., Tommasato G., Dellavia C., Del Fabbro M. Customized CAD/CAM Titanium Meshes for the Guided Bone Regeneration of Severe Alveolar Ridge Defects: Preliminary Results of a Retrospective Clinical Study in Humans. Clin. Oral Implant. Res. 2021;32:498–510. doi: 10.1111/clr.13720. [DOI] [PubMed] [Google Scholar]
- 86.Cucchi A., Vignudelli E., Sartori M., Parrilli A., Aldini N.N., Corinaldesi G. A Microcomputed Tomography Analysis of Bone Tissue after Vertical Ridge Augmentation with Non-Resorbable Membranes versus Resorbable Membranes and Titanium Mesh in Humans. Int. J. Oral Implant. 2021;14:25–38. [PubMed] [Google Scholar]
- 87.Cucchi A., Vignudelli E., Franceschi D., Randellini E., Lizio G., Fiorino A., Corinaldesi G. Vertical and Horizontal Ridge Augmentation Using Customized CAD/CAM Titanium Mesh with versus without Resorbable Membranes. A Randomized Clinical Trial. Clin. Oral Implant. Res. 2021;32:1411–1424. doi: 10.1111/clr.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Santis D., Gelpi F., Verlato G., Luciano U., Torroni L., Antonucci N., Bernardello F., Zarantonello M., Nocini P.F. Digital Customized Titanium Mesh for Bone Regeneration of Vertical, Horizontal and Combined Defects: A Case Series. Medicina. 2021;57:60. doi: 10.3390/medicina57010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dellavia C., Canciani E., Pellegrini G., Tommasato G., Graziano D., Chiapasco M. Histological Assessment of Mandibular Bone Tissue after Guided Bone Regeneration with Customized Computer-Aided Design/Computer-Assisted Manufacture Titanium Mesh in Humans: A Cohort Study. Clin. Implant. Dent. Relat. Res. 2021;23:600–611. doi: 10.1111/cid.13025. [DOI] [PubMed] [Google Scholar]
- 90.Kadkhodazadeh M., Amid R., Moscowchi A. Management of Extensive Peri-Implant Defects with Titanium Meshes. Oral Maxillofac. Surg. 2021;25:561–568. doi: 10.1007/s10006-021-00955-x. [DOI] [PubMed] [Google Scholar]
- 91.Li S., Zhao J., Xie Y., Tian T., Zhang T., Cai X. Hard Tissue Stability after Guided Bone Regeneration: A Comparison between Digital Titanium Mesh and Resorbable Membrane. Int. J. Oral. Sci. 2021;13:37. doi: 10.1038/s41368-021-00143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maiorana C., Fontana F., Dal Polo M.R., Pieroni S., Ferrantino L., Poli P.P., Simion M. Dense Polytetrafluoroethylene Membrane versus Titanium Mesh in Vertical Ridge Augmentation: Clinical and Histological Results of a Split-Mouth Prospective Study. J. Contemp. Dent. Pract. 2021;22:465–472. [PubMed] [Google Scholar]
- 93.Wang X., Wang G., Zhao X., Feng Y., Liu H., Li F. Short-Term Evaluation of Guided Bone Reconstruction with Titanium Mesh Membranes and CGF Membranes in Immediate Implantation of Anterior Maxillary Tooth. Biomed. Res. Int. 2021;2021:4754078. doi: 10.1155/2021/4754078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoon J.-H., Park Y.-W., Kim S.-G. Titanium Mesh and Pedicled Buccal Fat Pad for the Reconstruction of Maxillary Defect: Case Report. Maxillofac. Plast. Reconstr. Surg. 2021;43:10. doi: 10.1186/s40902-021-00295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bertran Faus A., Cordero Bayo J., Velasco-Ortega E., Torrejon-Moya A., Fernández-Velilla F., García F., López-López J. Customized Titanium Mesh for Guided Bone Regeneration with Autologous Bone and Xenograft. Materials. 2022;15:6271. doi: 10.3390/ma15186271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Del Barrio R.A.L., de Souza E.R., Al Houch A.O., Marão H.F. Rehabilitation of Severely Atrophic Mandible: A 3-Year Follow-Up Protocol. J. Oral. Implant. 2022;48:475–479. doi: 10.1563/aaid-joi-D-20-00418. [DOI] [PubMed] [Google Scholar]
- 97.Gelețu G.L., Burlacu A., Murariu A., Andrian S., Golovcencu L., Baciu E.-R., Maftei G., Onica N. Customized 3D-Printed Titanium Mesh Developed for an Aesthetic Zone to Regenerate a Complex Bone Defect Resulting after a Deficient Odontectomy: A Case Report. Medicina. 2022;58:1192. doi: 10.3390/medicina58091192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hartmann A., Hildebrandt H., Younan Z., Al-Nawas B., Kämmerer P.W. Long-Term Results in Three-Dimensional, Complex Bone Augmentation Procedures with Customized Titanium Meshes. Clin. Oral. Implant. Res. 2022;33:1171–1181. doi: 10.1111/clr.14000. [DOI] [PubMed] [Google Scholar]
- 99.Levine R.A., Lai P.-C., Manji A., Bruce J., Katwal D., Chen P.-H.A., Faucher J., McAllister B.S., Fava P., Scarfe W.C. Implant Site Development Using Titanium Mesh in the Maxilla: A Retrospective Study of 58 Mesh Procedures in 48 Patients. Int. J. Periodontics Restor. Dent. 2022;42:43–51. doi: 10.11607/prd.5530. [DOI] [PubMed] [Google Scholar]
- 100.Lim J., Jun S.H., Tallarico M., Park J.-B., Park D.-H., Hwang K.-G., Park C.-J. A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes. Materials. 2022;15:5294. doi: 10.3390/ma15155294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Majewski P. The Ti-Mesh Technique: Guided Bone Regeneration for Three-Dimensional Augmentations. Clinical Aspects: A Case Series. Int. J. Periodontics. Restor. Dent. 2022;42:145–153. doi: 10.11607/prd.5692. [DOI] [PubMed] [Google Scholar]
- 102.Müller J., Junker R. Customized Titanium Mesh for Guided Bone Regeneration in the Posterior Mandible in a Patient Previously Treated with Bisphosphonates. Case. Rep. Dent. 2022;2022:5174075. doi: 10.1155/2022/5174075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poomprakobsri K., Kan J.Y., Rungcharassaeng K., Lozada J., Oyoyo U. Exposure of Barriers Used in Guided Bone Regeneration: Rate, Timing, Management, and Effect on Grafted Bone-A Retrospective Analysis. J. Oral Implant. 2022;48:27–36. doi: 10.1563/aaid-joi-D-19-00252. [DOI] [PubMed] [Google Scholar]
- 104.Yang W., Chen D., Wang C., Apicella D., Apicella A., Huang Y., Li L., Zheng L., Ji P., Wang L., et al. The Effect of Bone Defect Size on the 3D Accuracy of Alveolar Bone Augmentation Performed with Additively Manufactured Patient-Specific Titanium Mesh. BMC Oral Health. 2022;22:557. doi: 10.1186/s12903-022-02557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Attia R., El-Nahass H., Anis M., Hosny M. Clinical Evaluation of Coronally Advanced Lingual Flap for Maintaining Primary Wound Closure over Titanium Mesh after Guided Bone Regeneration: A Randomized Control Trial. Int. J. Periodontics. Restor. Dent. 2023;43:s36–s52. doi: 10.11607/prd.6179. [DOI] [PubMed] [Google Scholar]
- 106.Bahaa S., Diab N., Zazou N., Darhous M., El Arab A.E., ElNahass H. Evaluation of Bone Gain in Horizontal Ridge Augmentation Using Titanium Mesh in Combination with Different Flap Advancement Techniques: A Randomized Clinical Trial. Int. J. Oral Maxillofac. Surg. 2023;52:379–387. doi: 10.1016/j.ijom.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 107.Nan X., Wang C., Li L., Ma X., Chen T., Huang Y. Application of Three-Dimensional Printing Individualized Titanium Mesh in Alveolar Bone Defects with Different Terheyden Classifications: A Retrospective Case Series Study. Clin. Oral Implant. Res. 2023;34:639–650. doi: 10.1111/clr.14062. [DOI] [PubMed] [Google Scholar]
- 108.Onică N., Onică C.A., Baciu E.-R., Vasluianu R.-I., Ciofu M., Balan M., Gelețu G.L. Advanced Techniques for Bone Restoration and Immediate Loading after Implant Failure: A Case Report. Healthcare. 2023;11:1608. doi: 10.3390/healthcare11111608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li S., Zhao Y., Tian T., Zhang T., Xie Y., Cai X. A Minimally Invasive Method for Titanium Mesh Fixation with Resorbable Sutures in Guided Bone Regeneration: A Retrospective Study. Clin. Implant. Dent. Relat. Res. 2023;25:87–98. doi: 10.1111/cid.13147. [DOI] [PubMed] [Google Scholar]
- 110.Wen S.-C., Fu J.-H., Wang H.-L. Effect of Deproteinized Bovine Bone Mineral at Implant Dehiscence Defects Grafted by the Sandwich Bone Augmentation Technique. Int. J. Periodontics. Restor. Dent. 2018;38:79–85. doi: 10.11607/prd.2931. [DOI] [PubMed] [Google Scholar]
- 111.Zhang G., Miao X., Lin H., Qi B., Wu Y. A Tooth-Supported Titanium Mesh Bending and Positioning Module for Alveolar Bone Augmentation and Improving Accuracy. J. Esthet. Restor. Dent. 2023;35:586–595. doi: 10.1111/jerd.13012. [DOI] [PubMed] [Google Scholar]
- 112.Poli P.P., Beretta M., Maiorana C., Souza F.Á., Bovio A., Manfredini M. Therapeutic Strategies in the Management of Nonresorbable Membrane and Titanium Mesh Exposures Following Alveolar Bone Augmentation: A Systematic Scoping Review. Int. J. Oral Maxillofac. Implant. 2022;37:250–269. doi: 10.11607/jomi.9286. [DOI] [PubMed] [Google Scholar]
- 113.Boyne P.J. Restoration of Osseous Defects in Maxillofacial Casualties. J. Am. Dent. Assoc. 1969;78:767–776. doi: 10.1016/S0002-8177(69)84023-7. [DOI] [PubMed] [Google Scholar]
- 114.Aceves-Argemí R., Roca-Millan E., González-Navarro B., Marí-Roig A., Velasco-Ortega E., López-López J. Titanium Meshes in Guided Bone Regeneration: A Systematic Review. Coatings. 2021;11:316. doi: 10.3390/coatings11030316. [DOI] [Google Scholar]
- 115.Rasia-dal Polo M., Poli P.-P., Rancitelli D., Beretta M., Maiorana C. Alveolar Ridge Reconstruction with Titanium Meshes: A Systematic Review of the Literature. Med. Oral Patol. Oral Cir. Bucal. 2014;19:e639–e646. doi: 10.4317/medoral.19998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All experimental data that support the findings of this study are available from the corresponding author upon request.