Abstract
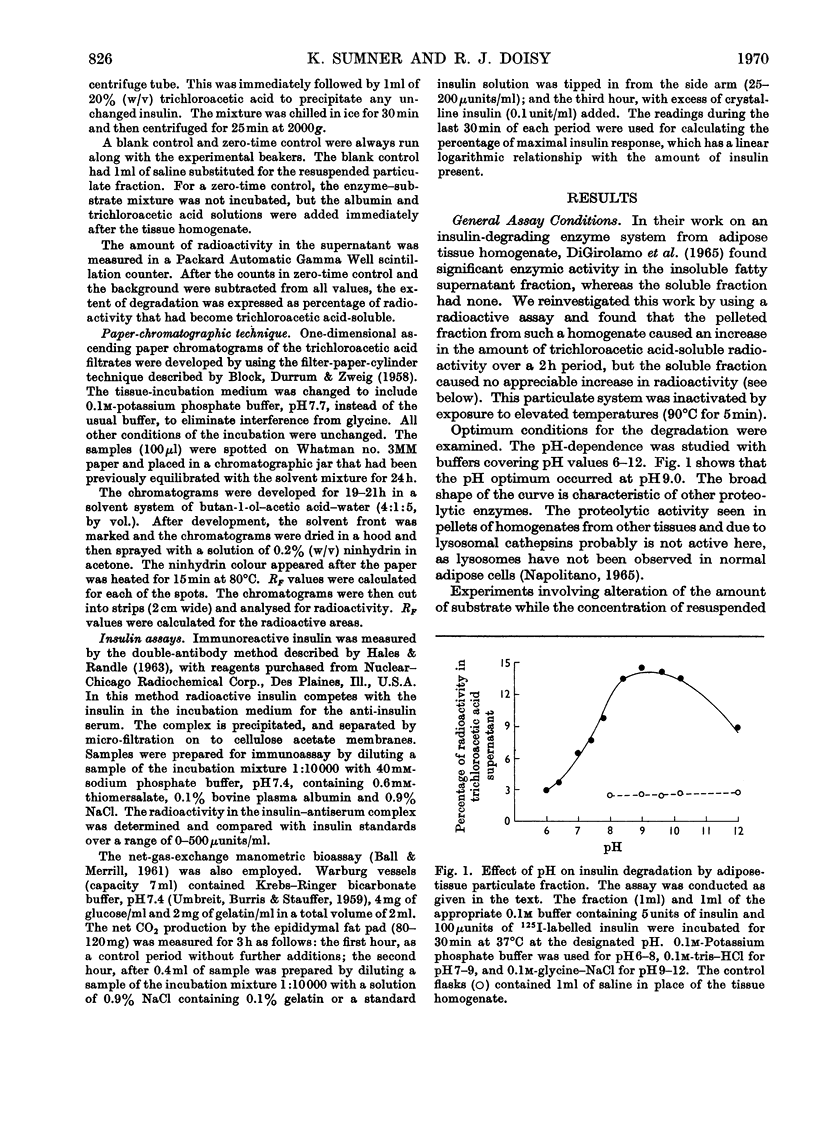
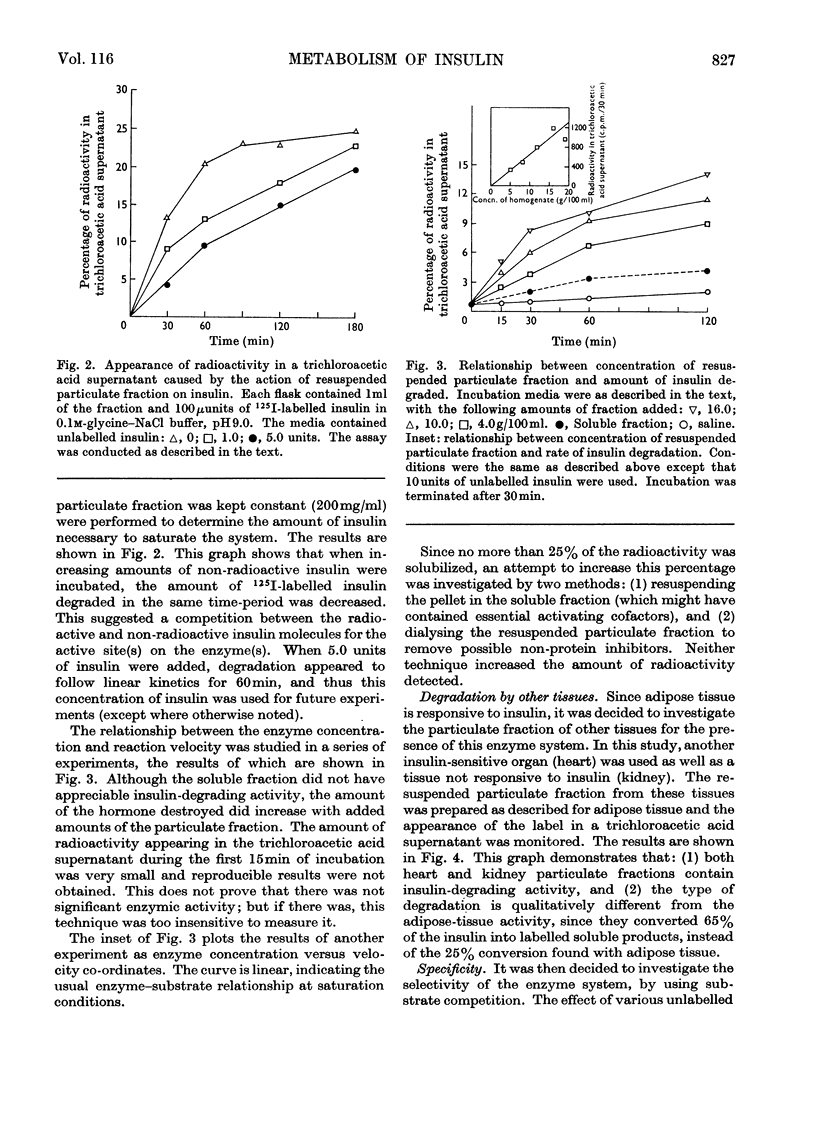
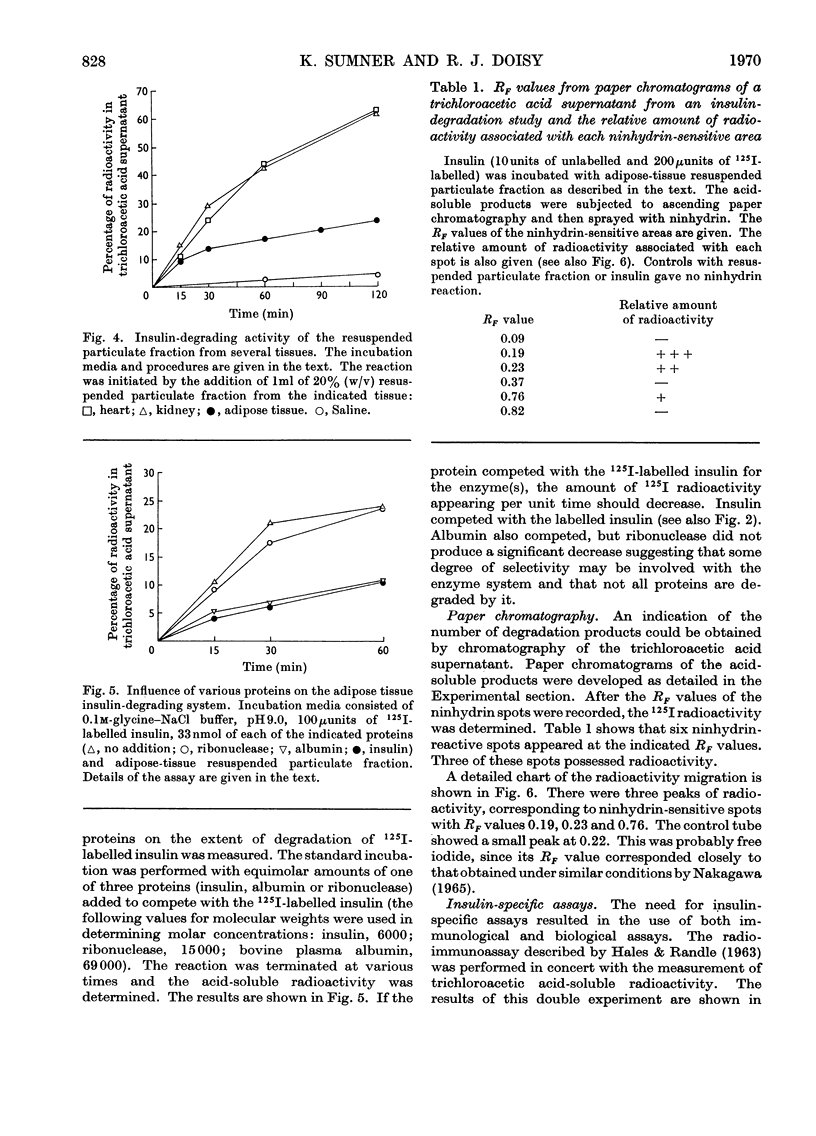
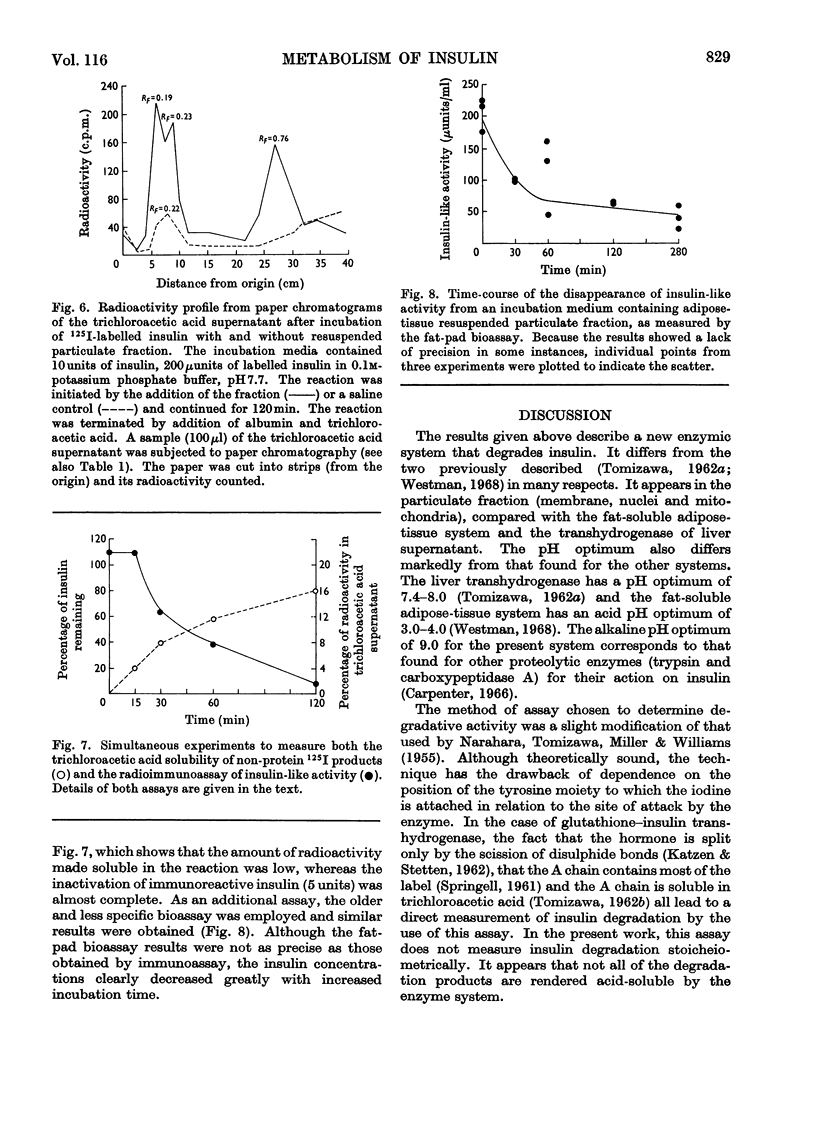
The destruction of 125I-labelled insulin by an enzyme system from rat adipose tissue was studied. The system was located in the particulate fraction. Activity was assayed by the amount of 125I-labelled degradation products rendered soluble in trichloroacetic acid. The system was heat-labile, with an alkaline pH optimum. The velocity of the reaction varied directly with the enzyme concentration. Paper chromatography of the degradation products showed six ninhydrin-sensitive areas with radioactivity coinciding with three of them. Albumin inhibited the system; ribonuclease did not. Although only 25% of the total 125I-label was detected by this assay, results with insulin-specific assays suggested that most (80–90%) of the hormone was inactivated. Possible interpretations of these results are discussed. The particulate fractions of other tissues were also studied.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALL E. G., MERRILL M. A. A manometric assay of insulin and some results of the application of the method to sera and islet-containing tissues. Endocrinology. 1961 Sep;69:596–607. doi: 10.1210/endo-69-3-596. [DOI] [PubMed] [Google Scholar]
- Carpenter F. H. Relationship of structure to biological activity of insulin as revealed by degradative studies. Am J Med. 1966 May;40(5):750–758. doi: 10.1016/0002-9343(66)90156-2. [DOI] [PubMed] [Google Scholar]
- Crofford O. B. The uptake and inactivation of native insulin by isolated fat cells. J Biol Chem. 1968 Jan 25;243(2):362–369. [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Garratt C. J., Jarrett R. J., Keen H. The relationship between insulin association with tissues and insulin action. Biochim Biophys Acta. 1966 May 26;121(1):143–150. doi: 10.1016/0304-4165(66)90357-6. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHARA H. T., TOMIZAWA H. H., MILLER R., WILLIAMS R. H. Intracellular distribution of an insulin-inactivating system of liver. J Biol Chem. 1955 Dec;217(2):675–684. [PubMed] [Google Scholar]
- SPRINGELL P. H. An unreactive tyrosine residue in insulin and the exclusive iodination of the A chain. Nature. 1961 Sep 30;191:1372–1373. doi: 10.1038/1911372a0. [DOI] [PubMed] [Google Scholar]
- TOMIZAWA H. H. Mode of action of an insulin-degrading enzyme from beef liver. J Biol Chem. 1962 Feb;237:428–431. [PubMed] [Google Scholar]
- TOMIZAWA H. H. Properties of glutathione insulin transhydrogenase from beef liver. J Biol Chem. 1962 Nov;237:3393–3396. [PubMed] [Google Scholar]
- Westman S. Degradation of insulin in vitro by liver and epididymal adipose tissue from obese-hyperglycaemic mice. Biochem J. 1968 Jan;106(2):543–547. doi: 10.1042/bj1060543. [DOI] [PMC free article] [PubMed] [Google Scholar]


