Abstract
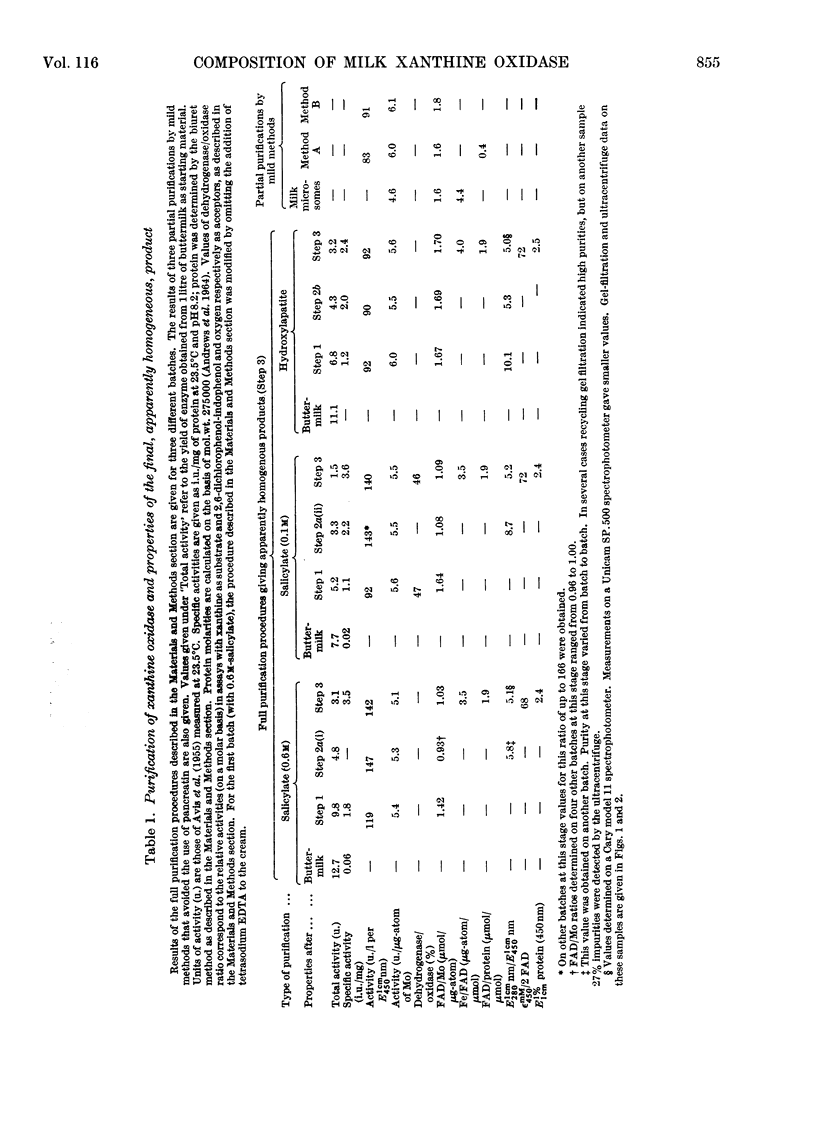
The composition of milk xanthine oxidase has been reinvestigated. When the enzyme is prepared by methods that include a selective denaturation step in the presence of sodium salicylate the product is obtained very conveniently and in high yield, and is homogeneous in the ultracentrifuge and in recycling gel filtration. It has specific activity higher than previously reported preparations of the enzyme and its composition approximates closely to 2mol of FAD, 2g-atoms of Mo and 8g-atoms of Fe/mol of protein (molecular weight about 275000). In contrast, when purely conventional preparative methods are used the product is also homogeneous by the above criteria but has a lower specific activity and is generally comparable to the crystallized enzyme described previously. Such samples also contain 2mol of FAD/mol of protein but they have lower contents of Mo (e.g. 1.2g-atom/mol). Amino acid compositions for the two types of preparation are indistinguishable. These results confirm the previous conclusion that conventional methods give mixtures of xanthine oxidase with an inactive modification of the enzyme now termed `de-molybdo-xanthine oxidase', and show that salicylate can selectively denature the latter. The origin of de-molybdo-xanthine oxidase was investigated. FAD/Mo ratios show that it is present not only in enzyme purified by conventional methods but also in `milk microsomes' (Bailie & Morton, 1958) and in enzyme samples prepared without proteolytic digestion. We conclude that it is secreted by cows together with the active enzyme and we discuss its occurrence in the preparations of other workers. Studies on the milks of individual cows show that nutritional rather than genetic factors determine the relative amounts of xanthine oxidase and de-molybdo-xanthine oxidase. A second inactive modification of the enzyme, now termed `inactivated xanthine oxidase', causes variability in activity relative to E450 or to Mo content and formation of it decreases these ratios during storage of enzyme samples including samples free from demolybdo-xanthine oxidase. We conclude that even the best purified xanthine oxidase samples described here and by other workers are contaminated by significant amounts of the inactivated form. This may complicate the interpretation of changes in the enzyme taking place during the slow phase of reduction by substrates. Attempts to remove iron from the enzyme by published methods were not successful.
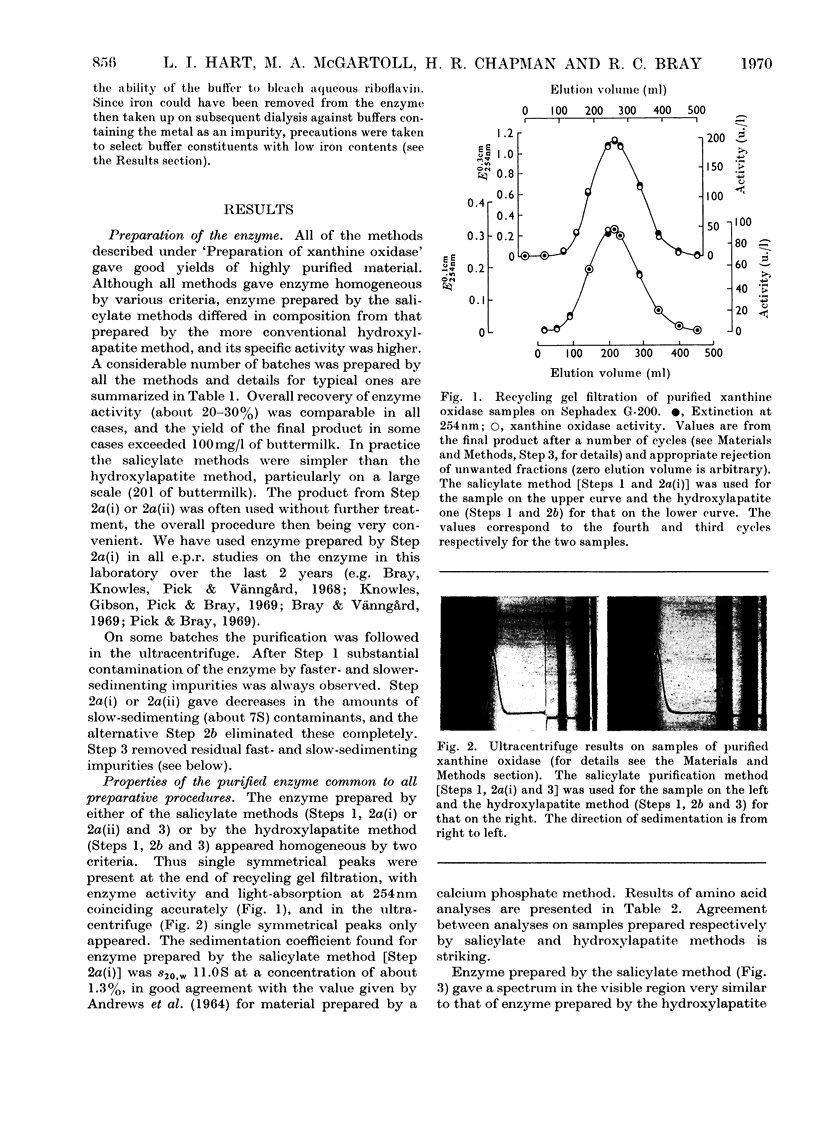
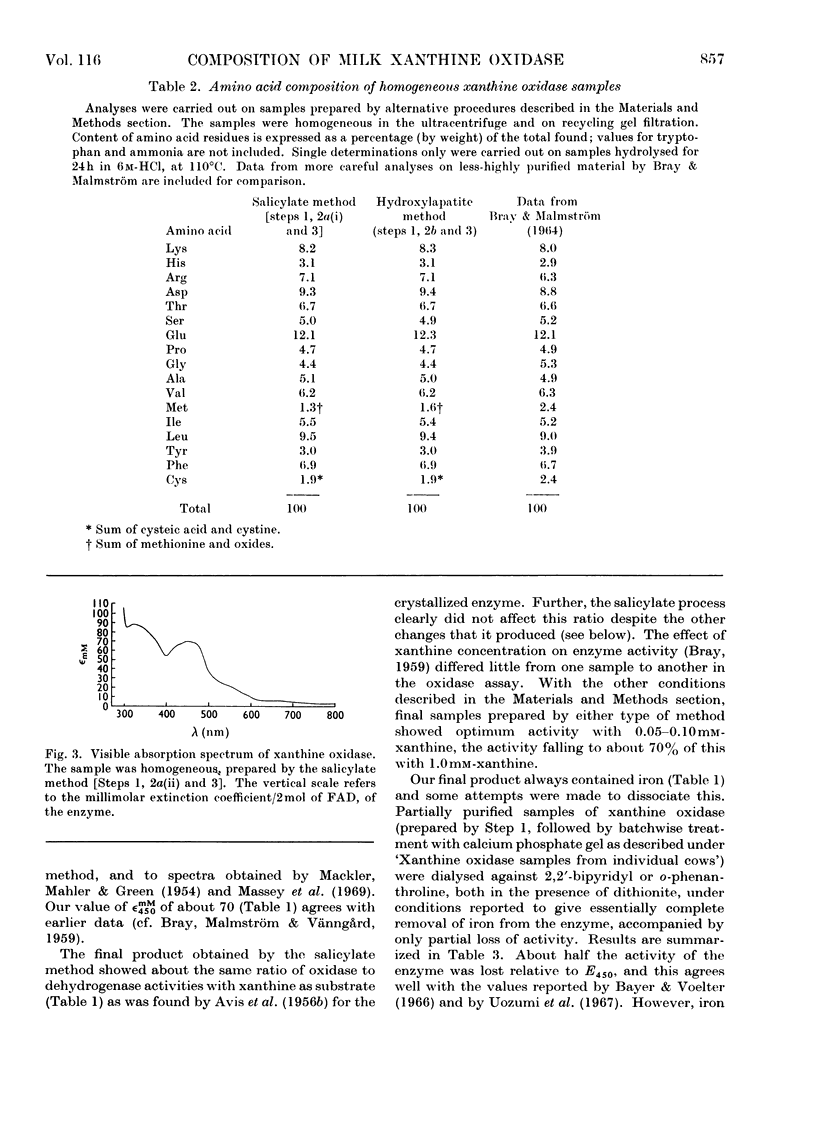
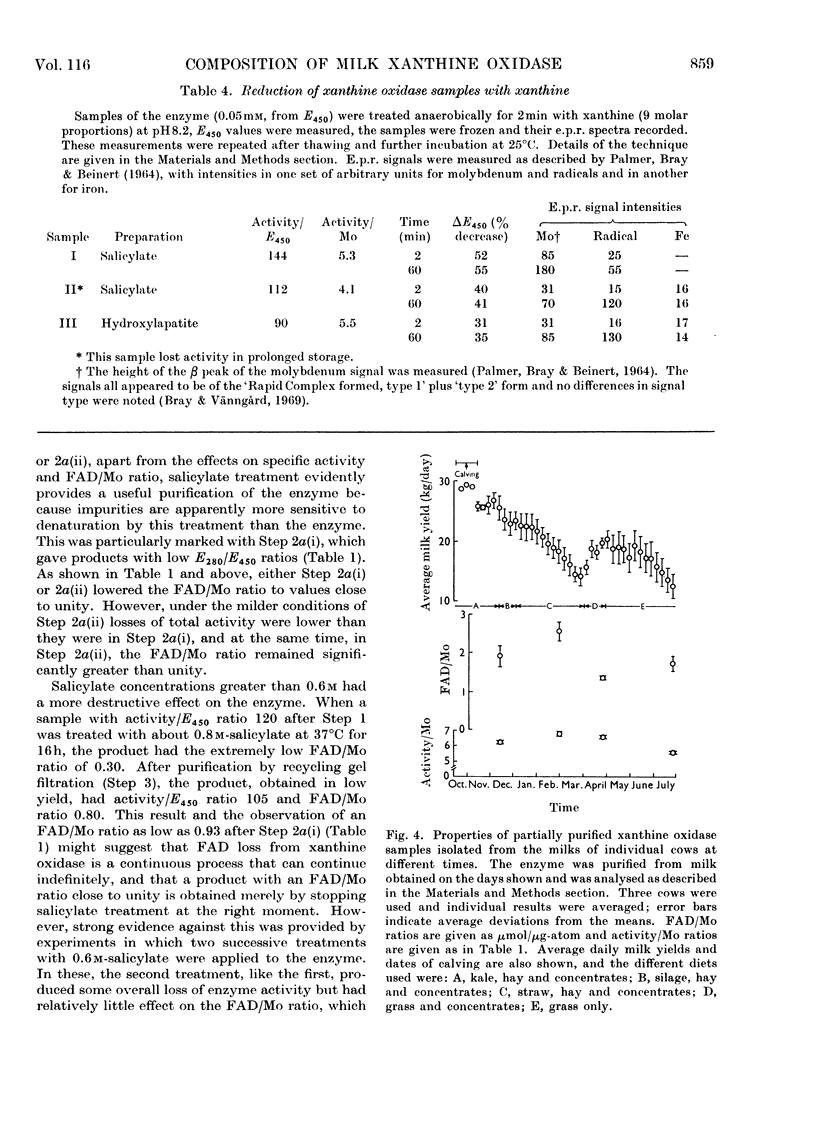
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P., Bray R. C., Edwards P., Shooter K. V. The chemistry of xanthine oxidase. 11. Ultracentrifuge and gel-filtration studies on the milk enzyme. Biochem J. 1964 Dec;93(3):627–632. doi: 10.1042/bj0930627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILIE M. J., MORTON R. K. Comparative properties of microsomes from cow's milk and from mammary gland. 1. Enzymic activities. Biochem J. 1958 May;69(1):35–44. doi: 10.1042/bj0690035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGEL F., BRAY R. C. The chemistry of xanthine oxidase. The problems of enzyme inactivation and stabilization. Biochem J. 1959 Sep;73:182–192. doi: 10.1042/bj0730182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAY R. C., MALMSTROM B. G., VANNGARD T. The chemistry of xanthine oxidase. Electron-spin resonance of xanthine oxidase solutions. Biochem J. 1959 Sep;73:193–197. doi: 10.1042/bj0730193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAY R. C., PALMER G., BEINERT H. DIRECT STUDIES ON THE ELECTRON TRANSFER SEQUENCE IN XANTHINE OXIDASE BY ELECTRON PARAMAGNETIC RESONANCE SPECTROSCOPY. II. KINETIC STUDIES EMPLOYING RAPID FREEZING. J Biol Chem. 1964 Aug;239:2667–2676. [PubMed] [Google Scholar]
- BRAY R. C., PETTERSSON R., EHRENBERG A. The chemistry of xanthine oxidase. 7. The anaerobic reduction of xanthine oxidase studied by electron-spin resonance and magnetic susceptibility. Biochem J. 1961 Oct;81:178–189. doi: 10.1042/bj0810178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAY R. C. The chemistry of xanthine oxidase. 6. Variations in stability and the presence of an inhibitor in certain preparations. Biochem J. 1959 Dec;73:690–694. doi: 10.1042/bj0730690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAY R. C. The chemistry of xanthine oxidase. 8. Electronspin-resonance measurements during the enzymic reaction. Biochem J. 1961 Oct;81:196–199. doi: 10.1042/bj0810196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E., Voelter W. Preparation of iron-free active xanthine oxidase. Biochim Biophys Acta. 1966 Mar 7;113(3):632–634. doi: 10.1016/s0926-6593(66)80027-9. [DOI] [PubMed] [Google Scholar]
- Bray R. C., Knowles P. F., Pick F. M., Vänngård T. Electron-spin-resonance evidence for interaction of protons with Mo(V) in reduced forms of xanthine oxidase. Biochem J. 1968 Apr;107(4):601–602. doi: 10.1042/bj1070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Malmström B. G. The chemistry of xanthine oxidase. 12. The amino acid composition. Biochem J. 1964 Dec;93(3):633–634. doi: 10.1042/bj0930633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Vänngård T. "Rapidly appearing" molybdenum electron-paramagnetic-resonance signals from reduced xanthine oxidase. Biochem J. 1969 Oct;114(4):725–734. doi: 10.1042/bj1140725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREY F. G., FRIDOVICH I., HANDLER P. Preparation of several forms of xanthine oxidase by enzymic proteolysis. Biochim Biophys Acta. 1961 Oct 28;53:440–442. doi: 10.1016/0006-3002(61)90468-1. [DOI] [PubMed] [Google Scholar]
- EHRENBERG A., BRAY R. C. MAGNETIC SUSCEPTIBILITY CHANGES AND ELECTRON SPIN RESONANCE SIGNALS RELATED TO THE IRON OF XANTHINE OXIDASE. Arch Biochem Biophys. 1965 Jan;109:199–202. doi: 10.1016/0003-9861(65)90309-7. [DOI] [PubMed] [Google Scholar]
- GILBERT D. A. Anaerobic reduction of bovine milk xanthine oxidase. Nature. 1963 Jun 22;198:1175–1177. doi: 10.1038/1981175a0. [DOI] [PubMed] [Google Scholar]
- GREEN D. E., BEINERT H. Xanthine oxidase, a molybdo-flavoprotein. Biochim Biophys Acta. 1953 Aug;11(4):599–600. doi: 10.1016/0006-3002(53)90111-5. [DOI] [PubMed] [Google Scholar]
- Gilbert D. A., Bergel F. The chemistry of xanthine oxidase. 9. An improved method of preparing the bovine milk enzyme. Biochem J. 1964 Feb;90(2):350–353. doi: 10.1042/bj0900350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart L. I., Bray R. C. Improved xanthine oxidase purification. Biochim Biophys Acta. 1967;146(2):611–613. doi: 10.1016/0005-2744(67)90253-7. [DOI] [PubMed] [Google Scholar]
- Knowles P. F., Gibson J. F., Pick F. M., Bray R. C. Electron-spin-resonance evidence for enzymic reduction of oxygen to a free radical, the superoxide ion. Biochem J. 1969 Jan;111(1):53–58. doi: 10.1042/bj1110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai H., Massey V., Palmer G. The preparation and properties of deflavo xanthine oxidase. J Biol Chem. 1969 Apr 10;244(7):1692–1700. [PubMed] [Google Scholar]
- MACKLER B., MAHLER H. R., GREEN D. E. Studies on metalloflavoproteins. I. Xanthine oxidase, a molybdoflavoprotein. J Biol Chem. 1954 Sep;210(1):149–164. [PubMed] [Google Scholar]
- MORELL D. B. The nature and catalytic activities of milk xanthine oxidase. Biochem J. 1952 Aug;51(5):657–666. doi: 10.1042/bj0510657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. Alkaline phosphatase of milk. 1. Association of the enzyme with a particulate lipoprotein complex. Biochem J. 1953 Dec;55(5):786–795. doi: 10.1042/bj0550786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Brumby P. E., Komai H. Studies on milk xanthine oxidase. Some spectral and kinetic properties. J Biol Chem. 1969 Apr 10;244(7):1682–1691. [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965 Nov;240(11):4470–4480. [PubMed] [Google Scholar]
- Nelson C. A., Handler P. Preparation of bovine xanthine oxidase and the subunit structures of some iron flavoproteins. J Biol Chem. 1968 Oct 25;243(20):5368–5373. [PubMed] [Google Scholar]
- PALMER G., BRAY R. C., BEINERT H. DIRECT STUDIES ON THE ELECTRON TRANSFER SEQUENCE IN XANTHINE OXIDASE BY ELECTRON PARAMAGNETIC RESONANCE SPECTROSCOPY. I. TECHNIQUES AND DESCRIPTION OF SPECTRA. J Biol Chem. 1964 Aug;239:2657–2666. [PubMed] [Google Scholar]
- PORATH J., BENNICH H. Recycling chromatography. Arch Biochem Biophys. 1962 Sep;Suppl 1:152–156. [PubMed] [Google Scholar]
- Pick F. M., Bray R. C. Complex-formation between reduced xanthine oxidase and purine substrates demonstrated by electron paramagnetic resonance. Biochem J. 1969 Oct;114(4):735–742. doi: 10.1042/bj1140735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHERT D. A., WESTERFELD W. W. The relationship of iron to xanthine oxidase. J Biol Chem. 1954 Jul;209(1):179–189. [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. The L-amino acid oxidases of snake venom. II. Isolation and characterization of homogeneous L-amino acid oxidase. Arch Biochem. 1950 Nov;29(1):190–209. [PubMed] [Google Scholar]
- TOTTER J. R., BURNETT W. T., Jr, MONROE R. A., WHITNEY I. B., COMAR C. L. Evidence that molybdenum is a nondialyzable component of xanthine oxidase. Science. 1953 Nov 6;118(3071):555–555. doi: 10.1126/science.118.3071.555. [DOI] [PubMed] [Google Scholar]
- Uozumi M., Hayashikawa R., Piette L. H. ESR and crystallization studies on iron-free xanthine oxidase. Arch Biochem Biophys. 1967 Mar;119(1):288–292. doi: 10.1016/0003-9861(67)90458-4. [DOI] [PubMed] [Google Scholar]



