Abstract
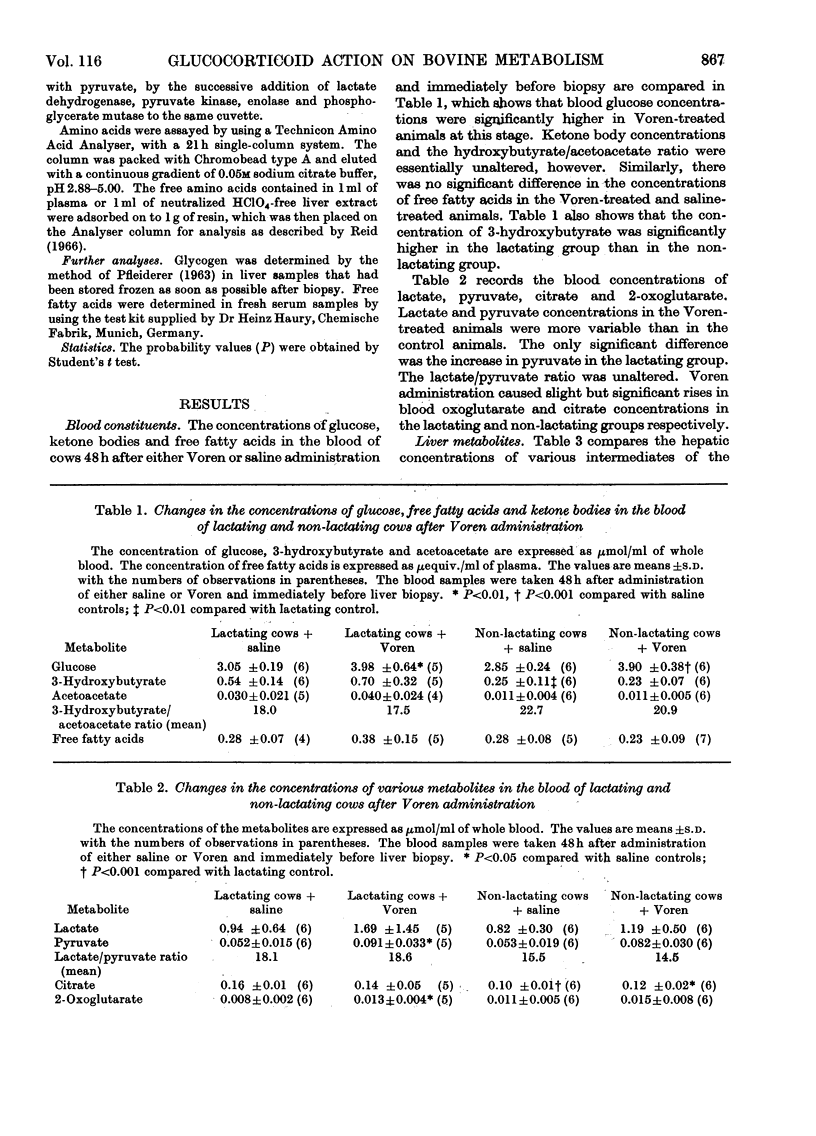
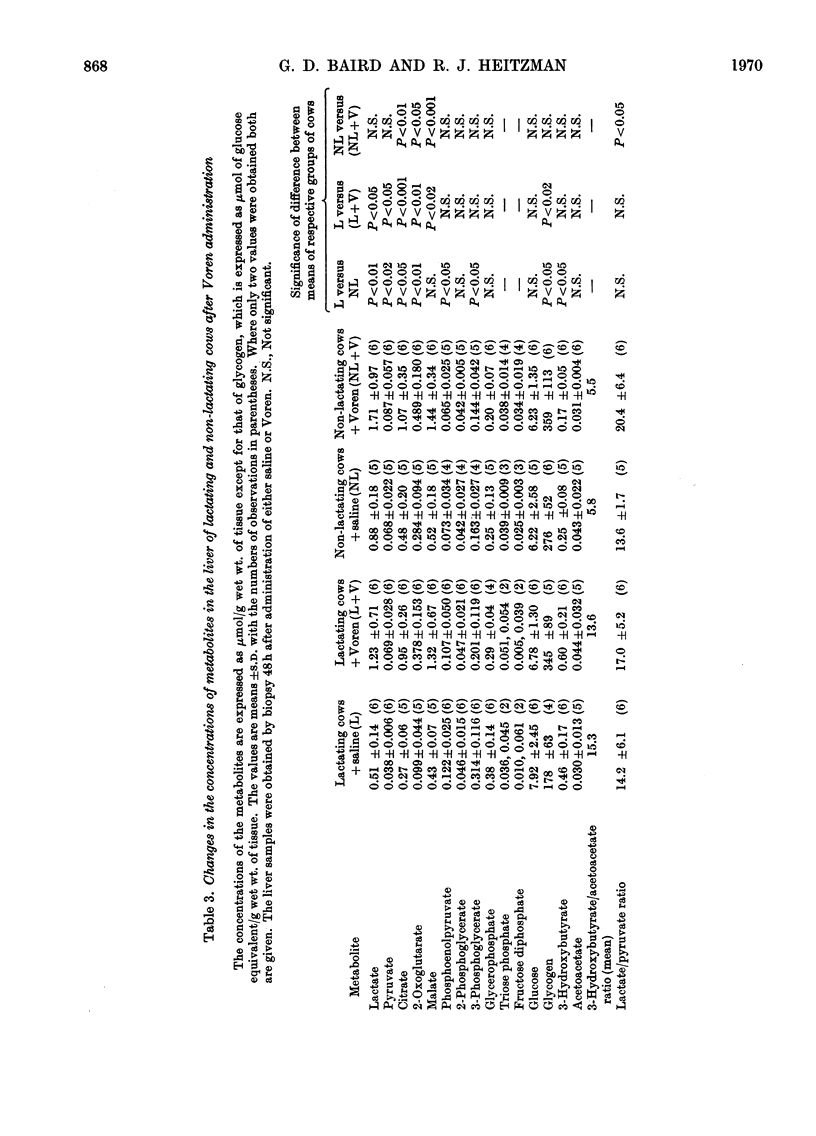
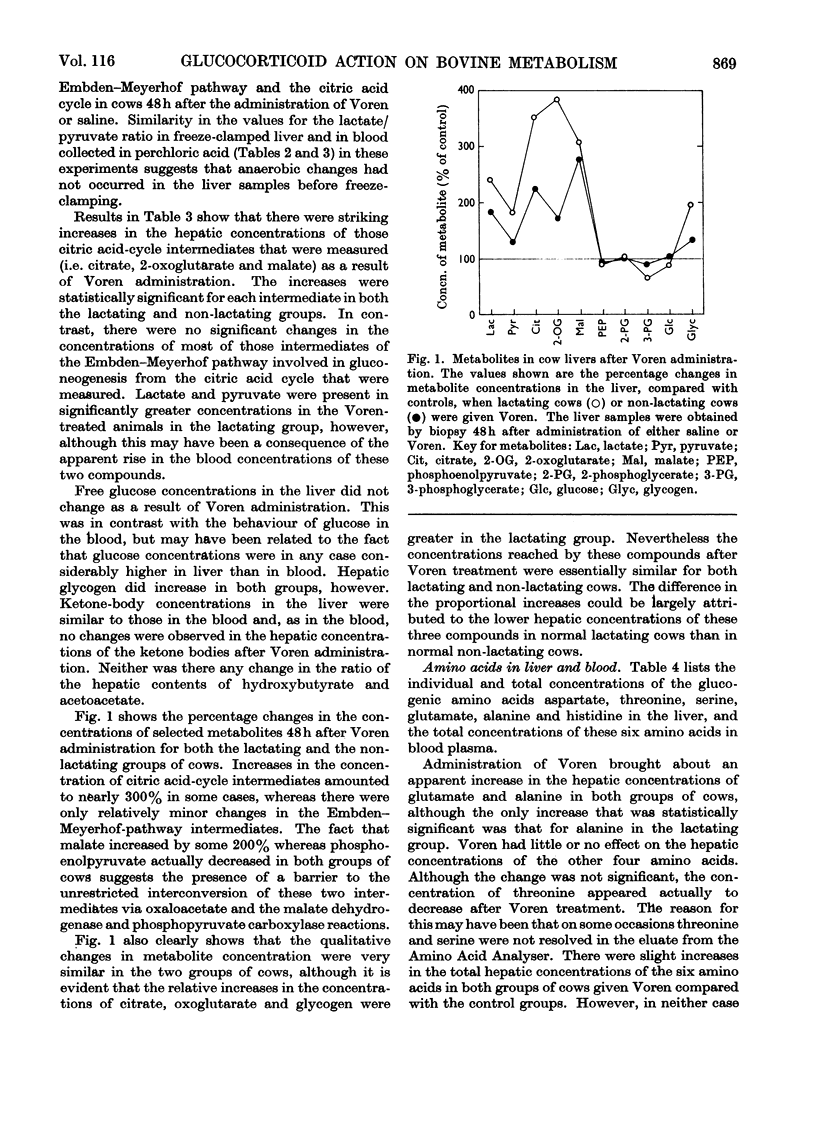
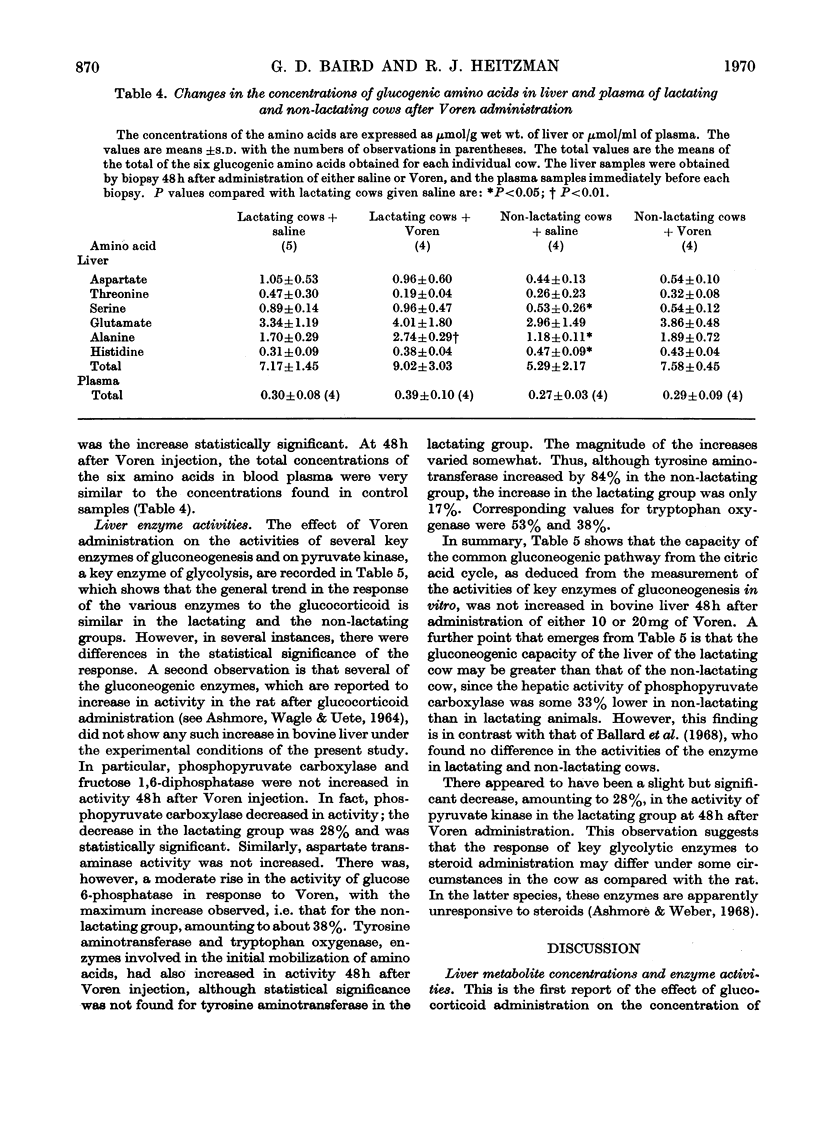
1. The hepatic concentrations of the ketone bodies and of metabolites and activities of enzymes involved in gluconeogenesis were measured in healthy lactating and non-lactating cows 48h after administration of Voren, an ester of dexamethasone, and compared with those found in control animals given saline. Parallel measurements were also made of the blood concentrations of several of the metabolites. 2. Blood glucose concentrations were raised in the Voren-treated animals, whereas blood ketone body and free fatty acid concentrations were unaltered. Similarly there was no change in the hepatic concentrations of the ketone bodies. 3. Significant increases were found in the hepatic concentrations of citrate, 2-oxo-glutarate and malate in both groups of animals given Voren. 4. The hepatic concentrations of those glycolytic intermediates that were measured either decreased or did not change after Voren treatment. 5. The enzymes aspartate transaminase and fructose 1,6-diphosphatase were unchanged in activity after Voren administration, whereas phosphopyruvate carboxylase (EC 4.1.1.32) activity was depressed in the lactating group. However, glucose 6-phosphatase, tryptophan oxygenase and tyrosine aminotransferase increased in activity. 6. In several cases those hepatic metabolites that increased in concentration after Voren administration were present in lower concentration in normal lactating cows than in normal non-lactating cows. The same applied mutatis mutandis to those metabolites that were decreased by Voren. 7. These findings are discussed in relation to the use of glucocorticoids in the treatment of bovine ketosis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J., Wagle S. R., Uete T. Studies on gluconeogenesis. Adv Enzyme Regul. 1964;2:101–114. doi: 10.1016/s0065-2571(64)80008-x. [DOI] [PubMed] [Google Scholar]
- Baird G. D. Fructose-1,6-diphosphatase and phosphopyruvate carboxykinase in bovine lactating mammary gland. Biochim Biophys Acta. 1969 Apr 1;177(2):343–345. doi: 10.1016/0304-4165(69)90145-7. [DOI] [PubMed] [Google Scholar]
- Baird G. D., Heitzman R. J. Antiketogenic action of glucocorticoids in the cow. Biochem J. 1969 Oct;114(4):69P–69P. doi: 10.1042/bj1140069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G. D., Hibbitt K. G., Hunter G. D. Biochemical aspects of bovine ketosis. Biochem J. 1968 May;107(5):683–689. doi: 10.1042/bj1070683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock D. J. Purification and properties of sheep liver phosphofructokinase. Biochem J. 1969 Jun;113(2):235–242. doi: 10.1042/bj1130235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filsell O. H., Jarrett I. G., Taylor P. H., Keech D. B. Effects of fasting, diabetes and glucocorticoids on gluconeogenic enzymes in the sheep. Biochim Biophys Acta. 1969 Jun 17;184(1):54–63. doi: 10.1016/0304-4165(69)90098-1. [DOI] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., REIM M., HUEBENER H. J. The oxidation/reduction state of the extramitochondrial DPN/DPNH system in rat liver and the hormonal control of substrate levels in vivo. Biochem Biophys Res Commun. 1961 Mar 10;4:163–168. doi: 10.1016/0006-291x(61)90263-7. [DOI] [PubMed] [Google Scholar]
- HORNBROOK K. R., BURCH H. B., LOWRY O. H. CHANGES IN SUBSTRATE LEVELS IN LIVER DURING GLYCOGEN SYNTHESIS INDUCED BY LACTATE AND HYDROCORTISONE. Biochem Biophys Res Commun. 1965 Jan 18;18:206–211. doi: 10.1016/0006-291x(65)90741-2. [DOI] [PubMed] [Google Scholar]
- Heitzman R. J. Hepatic enzyme activities early in lactation: a comparison of normal and ketotic cows. Res Vet Sci. 1969 Nov;10(6):582–584. [PubMed] [Google Scholar]
- Hibbitt K. G., Baird G. D. An induced ketosis and its role in the study of primary spontaneous bovine acetonaemia. Vet Rec. 1967 Nov 11;81(20):511–517. doi: 10.1136/vr.81.20.511. [DOI] [PubMed] [Google Scholar]
- Katz M. L., Bergman E. N. Hepatic and portal metabolism of glucose, free fatty acids, and ketone bodies in the sheep. Am J Physiol. 1969 Apr;216(4):953–960. doi: 10.1152/ajplegacy.1969.216.4.953. [DOI] [PubMed] [Google Scholar]
- Kreutner W., Goldberg N. D. Dependence on insulin of the apparent hydrocortisone activation of hepatic glycogen synthetase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1515–1519. doi: 10.1073/pnas.58.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matty A. J., Deutsch K. The ultrastructure of the thyroid of the hagfish, Myxine glutinosa. J Endocrinol. 1969 Sep;45(1 Suppl):xvii–xvii. [PubMed] [Google Scholar]
- Moellering H., Gruber W. Determination of citrate with citrate lyase. Anal Biochem. 1966 Dec;17(3):369–376. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- Seubert W., Huth W. On the mechanism of gluconeogenesis and its regulation. II. The mechanism of gluconeogenesis from pyruvate and fumarate. Biochem Z. 1965 Nov 15;343(2):176–191. [PubMed] [Google Scholar]
- WIELAND O., LOEFFLER G. UBER DEN MECHANISMUS DER GESTEIGERTEN KETONKOERPERBILDUNG. I. REDOX-STATUS DES LEBER-DPN UNTER KETOSEBEDINGUNGEN, IN VIVO. Biochem Z. 1963 Dec 3;339:204–211. [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]


