Abstract
Excitatory neurotransmitter is charged, so that emptying of a transmitter-containing vesicle (discharge) would seem to require considerable energy. Even if the energy problem is surmounted and discharge thereby made possible, there is still a problem of making the discharge fast enough (considerably less than 1 ms). Proposed here is a mechanism wherein discharge of charged transmitter is accompanied by the influx of cocharged ions or coefflux of counter-charged particles (ion interchange). It is shown theoretically that ion interchange obviates the necessity for a separate energy source and can provide the observed rapid vesicle discharge.
Full text
PDF



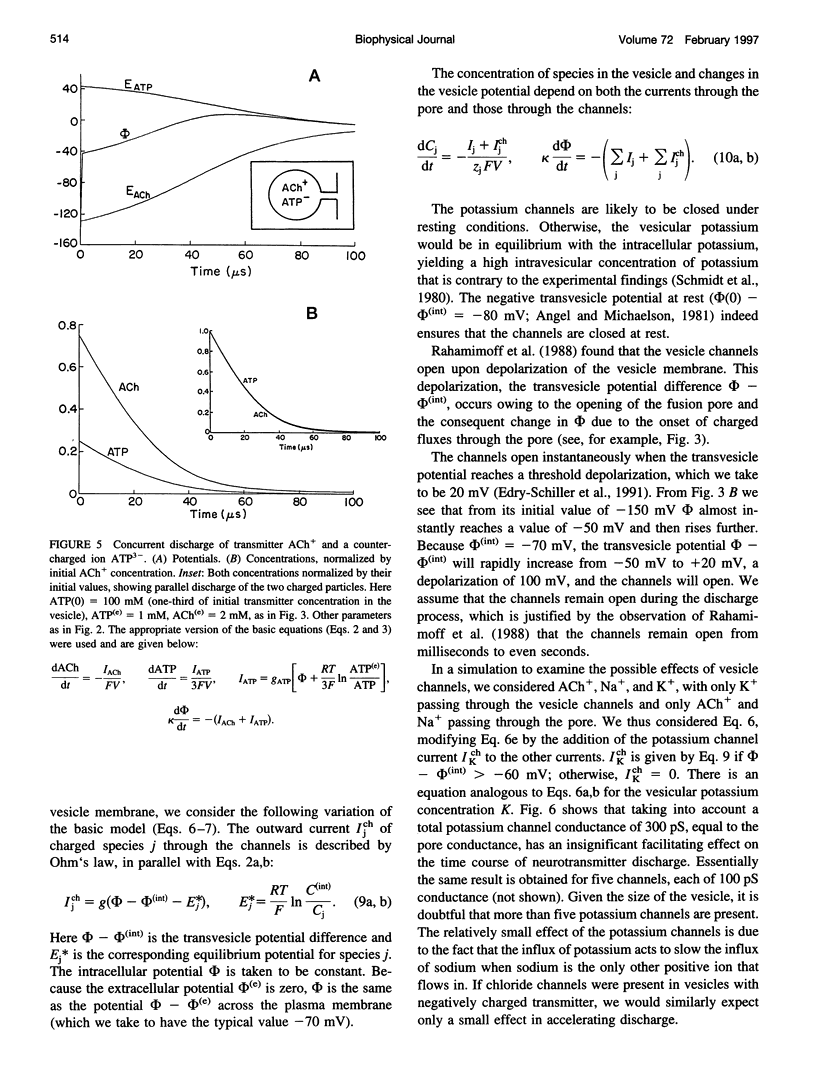
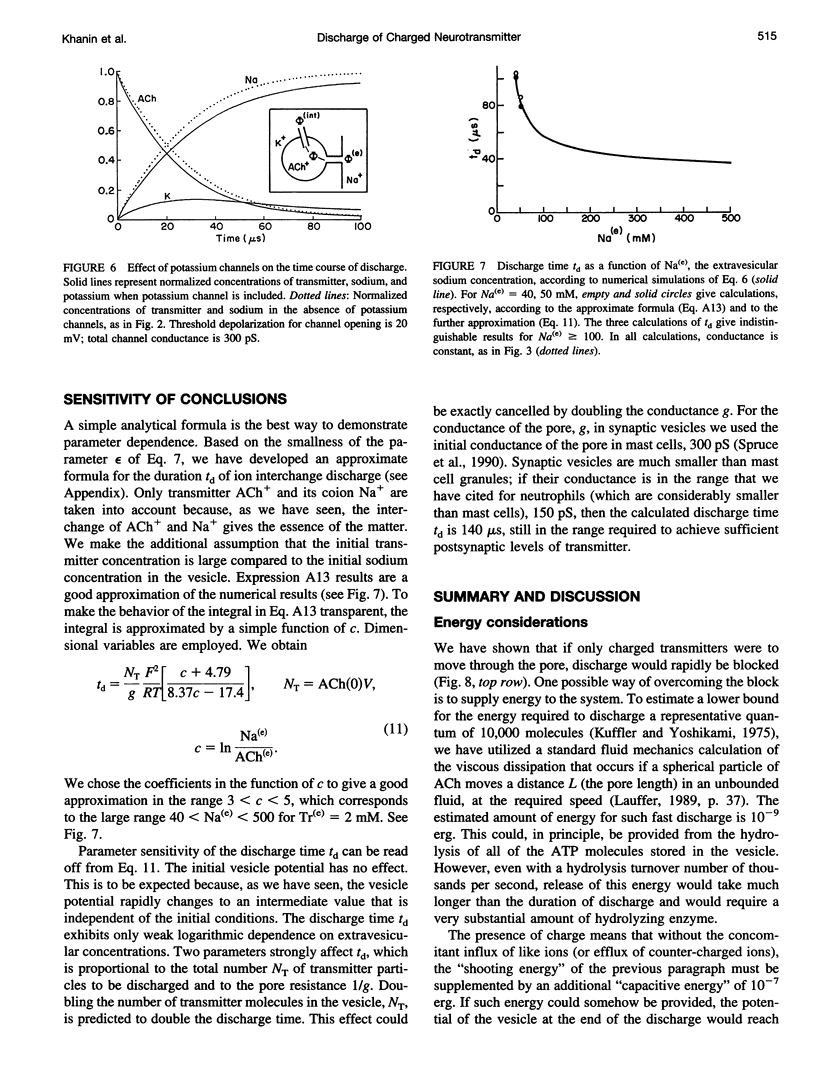

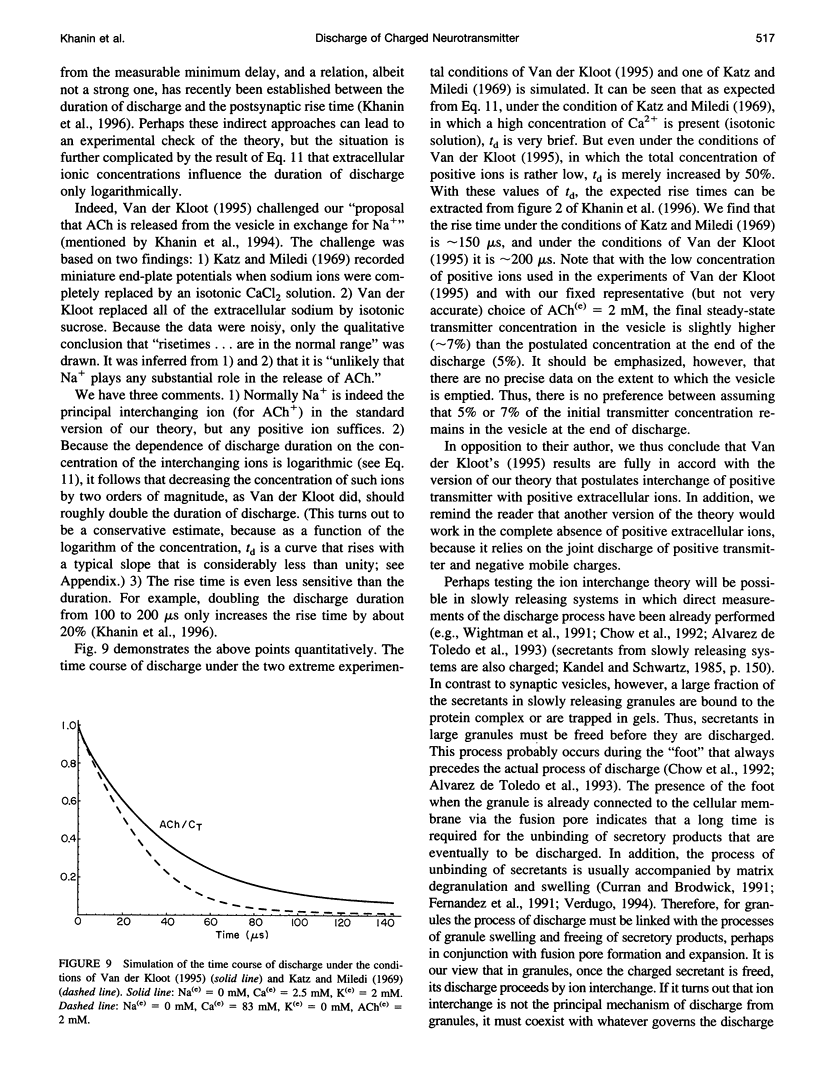




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Breckenridge L. J., Iwata A., Lee A. K., Spruce A. E., Tse F. W. Millisecond studies of single membrane fusion events. Ann N Y Acad Sci. 1991;635:318–327. doi: 10.1111/j.1749-6632.1991.tb36502.x. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G., Fernández-Chacón R., Fernández J. M. Release of secretory products during transient vesicle fusion. Nature. 1993 Jun 10;363(6429):554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Angel I., Michaelson D. M. Determination of delta psi, delta pH and the proton electrochemical gradient in isolated cholinergic synaptic vesicles. Life Sci. 1981 Jul 27;29(4):411–416. doi: 10.1016/0024-3205(81)90335-0. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. 1987 Aug 27-Sep 2Nature. 328(6133):814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Chow R. H., von Rüden L., Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992 Mar 5;356(6364):60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Curran M. J., Brodwick M. S. Ionic control of the size of the vesicle matrix of beige mouse mast cells. J Gen Physiol. 1991 Oct;98(4):771–790. doi: 10.1085/jgp.98.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran M. J., Cohen F. S., Chandler D. E., Munson P. J., Zimmerberg J. Exocytotic fusion pores exhibit semi-stable states. J Membr Biol. 1993 Apr;133(1):61–75. doi: 10.1007/BF00231878. [DOI] [PubMed] [Google Scholar]
- Edry-Schiller J., Ginsburg S., Rahamimoff R. A bursting potassium channel in isolated cholinergic synaptosomes of Torpedo electric organ. J Physiol. 1991 Aug;439:627–647. doi: 10.1113/jphysiol.1991.sp018685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Villalón M., Verdugo P. Reversible condensation of mast cell secretory products in vitro. Biophys J. 1991 May;59(5):1022–1027. doi: 10.1016/S0006-3495(91)82317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J., Lindau M. A novel Ca(2+)-dependent step in exocytosis subsequent to vesicle fusion. FEBS Lett. 1995 Apr 24;363(3):217–220. doi: 10.1016/0014-5793(95)00318-4. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium Ringer. J Physiol. 1969 Aug;203(3):689–706. doi: 10.1113/jphysiol.1969.sp008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanin R., Parnas H., Segel L. Diffusion cannot govern the discharge of neurotransmitter in fast synapses. Biophys J. 1994 Sep;67(3):966–972. doi: 10.1016/S0006-3495(94)80562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanin R., Segel L., Parnas H., Ratner E. Neurotransmitter discharge and postsynaptic rise times. Biophys J. 1996 Apr;70(4):2030–2032. doi: 10.1016/S0006-3495(96)79769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Salpeter E. E., Salpeter M. M. Acetylcholine receptor site density affects the rising phase of miniature endplate currents. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3736–3740. doi: 10.1073/pnas.77.6.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M., Nüsse O., Bennett J., Cromwell O. The membrane fusion events in degranulating guinea pig eosinophils. J Cell Sci. 1993 Jan;104(Pt 1):203–210. doi: 10.1242/jcs.104.1.203. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lollike K., Borregaard N., Lindau M. The exocytotic fusion pore of small granules has a conductance similar to an ion channel. J Cell Biol. 1995 Apr;129(1):99–104. doi: 10.1083/jcb.129.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews-Bellinger J., Salpeter M. M. Distribution of acetylcholine receptors at frog neuromuscular junctions with a discussion of some physiological implications. J Physiol. 1978 Jun;279:197–213. doi: 10.1113/jphysiol.1978.sp012340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monck J. R., Fernandez J. M. The exocytotic fusion pore. J Cell Biol. 1992 Dec;119(6):1395–1404. doi: 10.1083/jcb.119.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanavati C., Fernandez J. M. The secretory granule matrix: a fast-acting smart polymer. Science. 1993 Feb 12;259(5097):963–965. doi: 10.1126/science.8438154. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Blaustein M. P. GABA efflux from synaptosomes: effects of membrane potential, and external GABA and cations. J Membr Biol. 1982;69(3):213–223. doi: 10.1007/BF01870400. [DOI] [PubMed] [Google Scholar]
- Parnas H., Parnas I. Neurotransmitter release at fast synapses. J Membr Biol. 1994 Dec;142(3):267–279. doi: 10.1007/BF00233434. [DOI] [PubMed] [Google Scholar]
- Parsons S. M., Prior C., Marshall I. G. Acetylcholine transport, storage, and release. Int Rev Neurobiol. 1993;35:279–390. doi: 10.1016/s0074-7742(08)60572-3. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., DeRiemer S. A., Sakmann B., Stadler H., Yakir N. Ion channels in synaptic vesicles from Torpedo electric organ. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5310–5314. doi: 10.1073/pnas.85.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman R. S., Silinsky E. M. ATP released together with acetylcholine as the mediator of neuromuscular depression at frog motor nerve endings. J Physiol. 1994 May 15;477(Pt 1):117–127. doi: 10.1113/jphysiol.1994.sp020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Zimmermann H., Whittaker V. P. Metal ion content of cholinergic synaptic vesicles isolated from the electric organ of Torpedo: effect of stimulation-induced transmitter release. Neuroscience. 1980;5(3):625–638. doi: 10.1016/0306-4522(80)90060-3. [DOI] [PubMed] [Google Scholar]
- Spruce A. E., Breckenridge L. J., Lee A. K., Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990 May;4(5):643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- Stadler H., Füldner H. H. Proton NMR detection of acetylcholine status in synaptic vesicles. Nature. 1980 Jul 17;286(5770):293–294. doi: 10.1038/286293a0. [DOI] [PubMed] [Google Scholar]
- Stanley E. F., Ehrenstein G. A model for exocytosis based on the opening of calcium-activated potassium channels in vesicles. Life Sci. 1985 Nov 25;37(21):1985–1995. doi: 10.1016/0024-3205(85)90029-3. [DOI] [PubMed] [Google Scholar]
- Stiles J. R., Van Helden D., Bartol T. M., Jr, Salpeter E. E., Salpeter M. M. Miniature endplate current rise times less than 100 microseconds from improved dual recordings can be modeled with passive acetylcholine diffusion from a synaptic vesicle. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):5747–5752. doi: 10.1073/pnas.93.12.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torri-Tarelli F., Grohovaz F., Fesce R., Ceccarelli B. Temporal coincidence between synaptic vesicle fusion and quantal secretion of acetylcholine. J Cell Biol. 1985 Oct;101(4):1386–1399. doi: 10.1083/jcb.101.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs B., Aborg C. H. Cation exchange--a common mechanism in the storage and release of biogenic amines stored in granules (vesicles)? II. Comparative studies on sodium-induced release of biogenic amines from the synthetic weak cation-exchangers Amberlite IRC-50 and duolite CS-100 and from biogenic (granule-enriched) materials. Acta Physiol Scand. 1984 Jan;120(1):87–97. doi: 10.1111/j.1748-1716.1984.tb07377.x. [DOI] [PubMed] [Google Scholar]
- Uvnäs B., Aborg C. H. Cation exchange--a common mechanism in the storage and release of biogenic amines stored in granules (vesicles)? III. A possible role of sodium ions in non-exocytotic fractional release of neurotransmitters. Acta Physiol Scand. 1984 Jan;120(1):99–107. doi: 10.1111/j.1748-1716.1984.tb07378.x. [DOI] [PubMed] [Google Scholar]
- Uvnäs B., Aborg C. H., Lyssarides L., Thyberg J. Cation exchanger properties of isolated rat peritoneal mast cell granules. Acta Physiol Scand. 1985 Sep;125(1):25–31. doi: 10.1111/j.1748-1716.1985.tb07689.x. [DOI] [PubMed] [Google Scholar]
- Uvnäs B. An attempt to explain nervous transmitter release as due to nerve impulse-induced cation exchange. Acta Physiol Scand. 1973 Feb;87(2):168–175. doi: 10.1111/j.1748-1716.1973.tb05378.x. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. The rise times of miniature endplate currents suggest that acetylcholine may be released over a period of time. Biophys J. 1995 Jul;69(1):148–154. doi: 10.1016/S0006-3495(95)79884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. A., Carlson S. S., Kelly R. B. Chemical and physical characterization of cholinergic synaptic vesicles. Biochemistry. 1978 Apr 4;17(7):1199–1206. doi: 10.1021/bi00600a010. [DOI] [PubMed] [Google Scholar]
- Wahl L. M., Pouzat C., Stratford K. J. Monte Carlo simulation of fast excitatory synaptic transmission at a hippocampal synapse. J Neurophysiol. 1996 Feb;75(2):597–608. doi: 10.1152/jn.1996.75.2.597. [DOI] [PubMed] [Google Scholar]
- Wightman R. M., Jankowski J. A., Kennedy R. T., Kawagoe K. T., Schroeder T. J., Leszczyszyn D. J., Near J. A., Diliberto E. J., Jr, Viveros O. H. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Vogel S. S., Chernomordik L. V. Mechanisms of membrane fusion. Annu Rev Biophys Biomol Struct. 1993;22:433–466. doi: 10.1146/annurev.bb.22.060193.002245. [DOI] [PubMed] [Google Scholar]